
Back خلات أبيراتيرون Arabic ئەبیراتێرۆن ئاسەتەیت CKB Abirateronacetat German آبیراترون استات Persian Abirateron-acetát Hungarian Abiraterone acetate Italian アビラテロン酢酸エステル Japanese 아비라테론 아세테이트 Korean ଆବିରାଟେରୋନ ଆସେଟେଟ OR Abirateron asetat Turkish
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | a" bir a' ter one |
| Trade names | Zytiga, Yonsa, others |
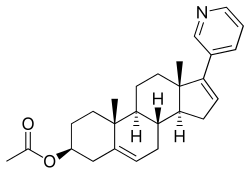
| Other names | CB-7630; JNJ-212082; 17-(3-Pyridinyl)androsta-5,16-dien-3β-ol acetate, abiraterone (BAN UK), abiraterone acetate (JAN JP), abiraterone acetate (USAN US) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a611046 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth[2][3] |
| Drug class | Antineoplastic |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Unknown, but may be 50% at most on empty stomach[6] |
| Protein binding | Abiraterone: ~99.8% (to albumin and α1-AGp)[6][2][7] |
| Metabolism | Esterases, CYP3A4, SULT2A1[7] |
| Metabolites | Abiraterone, others[2][6] |
| Elimination half-life | Abiraterone: 12–24 hours[2][6][3] |
| Excretion | Feces: 88%[2][7] Urine: 5%[2][7][3] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.149.063 |
| Chemical and physical data | |
| Formula | C26H33NO2 |
| Molar mass | 391.555 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 144 to 145 °C (291 to 293 °F) [8] |
| |
| |
| (verify) | |
Abiraterone acetate, sold under the brand name Zytiga among others, is a medication used to treat prostate cancer.[9] Specifically it is used together with a corticosteroid for metastatic castration-resistant prostate cancer (mCRPC) and metastatic high-risk castration-sensitive prostate cancer (mCSPC).[2][3] It should either be used following removal of the testicles or along with a gonadotropin-releasing hormone (GnRH) analog.[2] It is taken by mouth.[9]
Common side effects include tiredness, vomiting, headache, joint pain, high blood pressure, swelling, low blood potassium, high blood sugar, hot flashes, diarrhea, and cough.[9][2] Other severe side effects may include liver failure and adrenocortical insufficiency.[2] In males whose partners can become pregnant, birth control is recommended.[2] Supplied as abiraterone acetate it is converted in the body to abiraterone.[2] Abiraterone acetate works by suppressing the production of androgens – specifically it inhibits CYP17A1 – and thereby decreases the production of testosterone.[9] In doing so, it prevents the effects of these hormones in prostate cancer.[9]
Abiraterone acetate was described in 1995, and approved for medical use in the United States and the European Union in 2011.[10][2] It is on the World Health Organization's List of Essential Medicines.[11][12] It is available as a generic medication.[13]
- ^ "Abiraterone Use During Pregnancy". Drugs.com. 13 March 2020. Archived from the original on 25 November 2020. Retrieved 8 June 2020.
- ^ a b c d e f g h i j k l m n o "Zytiga- abiraterone acetate tablet, film coated". DailyMed. 13 June 2019. Archived from the original on 13 November 2014. Retrieved 15 November 2019.
- ^ a b c d e "Yonsa- abiraterone acetate tablet". DailyMed. 5 June 2018. Archived from the original on 13 August 2020. Retrieved 15 November 2019.
- ^ Cite error: The named reference
TGAwas invoked but never defined (see the help page). - ^ Cite error: The named reference
EMCwas invoked but never defined (see the help page). - ^ a b c d Benoist GE, Hendriks RJ, Mulders PF, Gerritsen WR, Somford DM, Schalken JA, van Oort IM, Burger DM, van Erp NP (November 2016). "Pharmacokinetic Aspects of the Two Novel Oral Drugs Used for Metastatic Castration-Resistant Prostate Cancer: Abiraterone Acetate and Enzalutamide". Clin Pharmacokinet. 55 (11): 1369–1380. doi:10.1007/s40262-016-0403-6. PMC 5069300. PMID 27106175.
- ^ a b c d "Meeting Library - Meeting Library". meetinglibrary.asco.org. Archived from the original on 20 September 2016. Retrieved 9 September 2016.
- ^ Potter GA, Barrie SE, Jarman M, Rowlands MG (June 1995). "Novel steroidal inhibitors of human cytochrome P45017 alpha (17 alpha-hydroxylase-C17,20-lyase): potential agents for the treatment of prostatic cancer". Journal of Medicinal Chemistry. 38 (13): 2463–2471. doi:10.1021/jm00013a022. PMID 7608911.
- ^ a b c d e "Abiraterone Acetate Monograph for Professionals". Drugs.com. Archived from the original on 6 May 2012. Retrieved 15 November 2019.
- ^ Scowcroft H (21 September 2011). "Where did abiraterone come from?". Journal of Medicinal Chemistry. 38 (13). Cancer Research UK: 2463–2471. Archived from the original on 25 September 2011. Retrieved 28 September 2011.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ "First Generic Drug Approvals". U.S. Food and Drug Administration. 17 October 2022. Retrieved 28 November 2022.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search