
Back أكامبروسيت Arabic Acamprosat German Acamprosato Spanish آکامپروسات Persian Acamprosate French अकेम्प्रोसेट Hindi Acamprosato Italian アカンプロサート Japanese Acamprosaat Dutch ଏକାମ୍ପ୍ରୋସେଟ OR
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /əˈkæmproʊseɪt/ |
| Trade names | Campral EC |
| Other names | N-Acetyl homotaurine, acamprosate calcium (JAN JP), acamprosate calcium (USAN US) |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Oral[1] |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 11%[1] |
| Protein binding | Negligible[1] |
| Metabolism | Nil[1] |
| Elimination half-life | 20 h to 33 h[1] |
| Excretion | Kidney[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.071.495 |
| Chemical and physical data | |
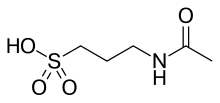
| Formula | C5H11NO4S |
| Molar mass | 181.21 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Acamprosate, sold under the brand name Campral, is a medication which reduces alcoholism cravings.[1][3] It is thought to stabilize chemical signaling in the brain that would otherwise be disrupted by alcohol withdrawal.[4] When used alone, acamprosate is not an effective therapy for alcohol use disorder in most individuals,[5] as it only addresses withdrawal symptoms and not psychological dependence. It facilitates a reduction in alcohol consumption as well as full abstinence when used in combination with psychosocial support or other drugs that address the addictive behavior.[3][6][7]
Serious side effects include allergic reactions, abnormal heart rhythms, and low or high blood pressure, while less serious side effects include headaches, insomnia, and impotence.[8] Diarrhea is the most common side-effect.[9] It is unclear if use is safe during pregnancy.[10][11]
It is on the World Health Organization's List of Essential Medicines.[12]
- ^ a b c d e f g "Campral label" (PDF). FDA. January 2012. Retrieved 27 November 2017. For label updates see FDA index page for NDA 021431
- ^ "Prescription medicines: registration of new generic medicines and biosimilar medicines, 2017". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 30 March 2024.
- ^ a b Plosker GL (July 2015). "Acamprosate: A Review of Its Use in Alcohol Dependence". Drugs. 75 (11): 1255–1268. doi:10.1007/s40265-015-0423-9. PMID 26084940. S2CID 19119078.
- ^ Williams SH (November 2005). "Medications for treating alcohol dependence". American Family Physician. 72 (9): 1775–1780. PMID 16300039. Archived from the original on 2007-09-29. Retrieved 2006-11-29.
- ^ Malenka RC, Nestler EJ, Hyman SE, Holtzman DM (2015). "Chapter 16: Reinforcement and Addictive Disorders". Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (3rd ed.). New York: McGraw-Hill Medical. ISBN 978-0-07-182770-6.
It has been hypothesized that long-term ethanol exposure alters the expression or activity of specific GABAA receptor subunits in discrete brain regions. Regardless of the underlying mechanism, ethanol-induced decreases in GABAA receptor sensitivity are believed to contribute to ethanol tolerance, and also may mediate some aspects of physical dependence on ethanol. ... Detoxification from ethanol typically involves the administration of benzodiazepines such as chlordiazepoxide, which exhibit cross-dependence with ethanol at GABAA receptors (Chapters 5 and 15). A dose that will prevent the physical symptoms associated with withdrawal from ethanol, including tachycardia, hypertension, tremor, agitation, and seizures, is given and is slowly tapered. Benzodiazepines are used because they are less reinforcing than ethanol among alcoholics. Moreover, the tapered use of a benzodiazepine with a long half-life makes the emergence of withdrawal symptoms less likely than direct withdrawal from ethanol. ... Unfortunately, acamprosate is not adequately effective for most alcoholics.
- ^ Mason BJ (2001). "Treatment of alcohol-dependent outpatients with acamprosate: a clinical review". The Journal of Clinical Psychiatry. 62 (Suppl 20): 42–48. PMID 11584875.
- ^ Nutt DJ, Rehm J (January 2014). "Doing it by numbers: a simple approach to reducing the harms of alcohol". Journal of Psychopharmacology. 28 (1): 3–7. doi:10.1177/0269881113512038. PMID 24399337. S2CID 36860967.
- ^ "Acamprosate". drugs.com. 2005-03-25. Archived from the original on 22 December 2006. Retrieved 2007-01-08.
- ^ Wilde MI, Wagstaff AJ (June 1997). "Acamprosate. A review of its pharmacology and clinical potential in the management of alcohol dependence after detoxification". Drugs. 53 (6): 1038–1053. doi:10.2165/00003495-199753060-00008. PMID 9179530. S2CID 195691152.
- ^ "Acamprosate (Campral) Use During Pregnancy". Drugs.com.
- ^ Haber P, Lintzeris N, Proude E, Lopatko O. "Guidelines for the Treatment of Alcohol Problems" (PDF). Australian Government Department of Health and Ageing. Retrieved 20 February 2023.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search