
Back أسيتات الأمونيوم Arabic آمونیوم استات AZB Acetat d'amoni Catalan Octan amonný Czech Ammoniumacetat German Amonia acetato Esperanto آمونیوم استات Persian Ammoniumasetaatti Finnish Acétate d'ammonium French Ammónium-acetát Hungarian
| |

| |

| |
| Names | |
|---|---|
| IUPAC name
Ammonium ethanoate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.010.149 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3077 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H7NO2 | |
| Molar mass | 77.083 g·mol−1 |
| Appearance | White solid crystals, deliquescent |
| Odor | Slightly acetic acid like |
| Density | 1.17 g/cm3 (20 °C)[1] 1.073 g/cm3 (25 °C) |
| Melting point | 113 °C (235 °F; 386 K)[4] |
| 102 g/100 mL (0 °C) 148 g/100 mL (4 °C)[1] 143 g/100 mL (20 °C) 533 g/100 mL (80 °C) | |
| Solubility | Soluble in alcohol, SO2, acetone, liquid ammonia[2] |
| Solubility in methanol | 7.89 g/100 mL (15 °C)[3][1] 131.24 g/100 g (94.2 °C)[2] |
| Solubility in dimethylformamide | 0.1 g/100 g[2] |
| Acidity (pKa) | 9.9 |
| Basicity (pKb) | 33 |
| -41.1·10−6 cm3/mol | |
| Viscosity | 21 |
| Structure | |
| Orthorhombic | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
−615 kJ/mol[2] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Irritant |
| GHS labelling:[3] | |

| |
| Warning | |
| H303, H316, H320, H333 | |
| P281, P335 | |
| NFPA 704 (fire diamond) | |
| Flash point | 136 °C (277 °F; 409 K)[3] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
386 mg/kg (mice, intravenous)[2] |
| Safety data sheet (SDS) | JT Baker |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
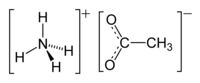
Ammonium acetate, also known as spirit of Mindererus in aqueous solution, is a chemical compound with the formula NH4CH3CO2. It is a white, hygroscopic solid and can be derived from the reaction of ammonia and acetic acid. It is available commercially.[5]
- ^ a b c Pradyot, Patnaik (2003). Handbook of Inorganic Chemicals. The McGraw-Hill Companies, Inc. ISBN 0-07-049439-8.
- ^ a b c d e "Ammonium acetate".
- ^ a b c d "Safety Data Sheet of Ammonium Acetate" (PDF). tedia.com. Tedia Company Inc. 2011-08-12. Retrieved 2014-06-10.
- ^ Davidson, Arthur W.; McAllister, Walter H. (1930). "Solutions of Salts in Pure Acetic Acid. Ii. Solubilities of Acetates1". Journal of the American Chemical Society. 52 (2): 507–519. doi:10.1021/ja01365a010. ISSN 0002-7863.
- ^ Hosea Cheung; Robin S. Tanke; G. Paul Torrence. "Acetic Acid". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_045.pub2. ISBN 978-3527306732.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search
