
Back Tellurid antimonitý Czech Antimon(III)-tellurid German Tellurure d'antimoine French 三テルル化二アンチモン Japanese Теллурид сурьмы(III) Russian Antimon telurid Serbo-Croatian Antimon telurid Serbian ஆண்டிமனி தெலூரைடு Tamil 三碲化二锑 Chinese
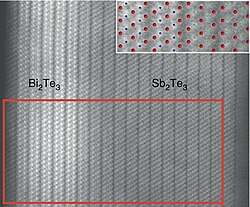 Electron micrograph of a seamless Bi2Te3/Sb2Te3 heterojunction and its atomic model (blue: Bi, green: Sb, red: Te)[1]
| |
| Names | |
|---|---|
| Other names
antimony telluride, antimony(III) telluride, antimony telluride, diantimony tritelluride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.014.074 |
PubChem CID
|
|
| |
| |
| Properties | |
| Sb2Te3 | |
| Molar mass | 626.32 g·mol−1 |
| Appearance | grey solid |
| Density | 6.50 g cm−3[2][3] |
| Melting point | 620 °C (1,148 °F; 893 K)[2] |
| Band gap | 0.21 eV[4] |
| Thermal conductivity | 1.65 W/(m·K) (308 K)[5] |
| Structure | |
| Rhombohedral, hR15 | |
| R3m, No. 166[6] | |
a = 0.4262 nm, c = 3.0435 nm
| |
Formula units (Z)
|
3 |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.5 mg/m3 (as Sb)[7] |
REL (Recommended)
|
TWA 0.5 mg/m3 (as Sb)[7] |
| Related compounds | |
Other anions
|
Sb2O3 Sb2S3 Sb2Se3 |
Other cations
|
As2Te3 Bi2Te3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Antimony telluride is an inorganic compound with the chemical formula Sb2Te3. As is true of other pnictogen chalcogenide layered materials, it is a grey crystalline solid with layered structure. Layers consist of two atomic sheets of antimony and three atomic sheets of tellurium and are held together by weak van der Waals forces. Sb2Te3 is a narrow-gap semiconductor with a band gap 0.21 eV; it is also a topological insulator, and thus exhibits thickness-dependent physical properties.[1]
- ^ a b Eschbach, Markus; Młyńczak, Ewa; Kellner, Jens; Kampmeier, Jörn; Lanius, Martin; Neumann, Elmar; Weyrich, Christian; Gehlmann, Mathias; Gospodarič, Pika; Döring, Sven; Mussler, Gregor; Demarina, Nataliya; Luysberg, Martina; Bihlmayer, Gustav; Schäpers, Thomas; Plucinski, Lukasz; Blügel, Stefan; Morgenstern, Markus; Schneider, Claus M.; Grützmacher, Detlev (2015). "Realization of a vertical topological p–n junction in epitaxial Sb2Te3/Bi2Te3 heterostructures". Nature Communications. 6: 8816. arXiv:1510.02713. Bibcode:2015NatCo...6.8816E. doi:10.1038/ncomms9816. PMC 4660041. PMID 26572278.
- ^ a b Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.48. ISBN 1-4398-5511-0.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 581–582. ISBN 978-0-08-037941-8.
- ^ Lefebvre, I.; Lannoo, M.; Allan, G.; Ibanez, A.; Fourcade, J.; Jumas, J. C.; Beaurepaire, E. (1987). "Electronic Properties of Antimony Chalcogenides". Physical Review Letters. 59 (21): 2471–2474. Bibcode:1987PhRvL..59.2471L. doi:10.1103/PhysRevLett.59.2471. PMID 10035559.
- ^ Yáñez-Limón, J. M.; González-Hernández, J.; Alvarado-Gil, J. J.; Delgadillo, I.; Vargas, H. (1995). "Thermal and electrical properties of the Ge:Sb:Te system by photoacoustic and Hall measurements". Physical Review B. 52 (23): 16321–16324. Bibcode:1995PhRvB..5216321Y. doi:10.1103/PhysRevB.52.16321. PMID 9981020.
- ^ Kim, Won-Sa (1997). "Solid state phase equilibria in the Pt–Sb–Te system". Journal of Alloys and Compounds. 252 (1–2): 166–171. doi:10.1016/S0925-8388(96)02709-0.
- ^ a b NIOSH Pocket Guide to Chemical Hazards. "#0036". National Institute for Occupational Safety and Health (NIOSH).
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search