
Back بنزوکتامین Persian Benzoktamin Serbo-Croatian Benzoktamin Serbian Bensoktamin Swedish Benzoctamine Vietnamese 苯佐他明 Chinese
 | |
| Clinical data | |
|---|---|
| Trade names | Tacitin |
| Routes of administration | Oral, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% for intravenous, 90% for oral |
| Metabolism | Hepatic |
| Elimination half-life | 2 to 3 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
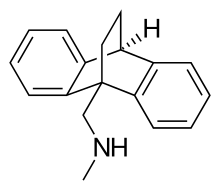
| Formula | C18H19N |
| Molar mass | 249.357 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Benzoctamine is a drug that possesses sedative and anxiolytic properties. Marketed as Tacitin by Ciba-Geigy, it is different from most sedative drugs because in most clinical trials it does not produce respiratory depression, but actually stimulates the respiratory system. As a result, when compared to other sedative and anxiolytic drugs such as benzodiazepines like diazepam, it is a safer form of tranquilizing. However, when co-administered with other drugs that cause respiratory depression, like morphine, it can cause increased respiratory depression.
Medically, benzoctamine is used as a treatment for anxious outpatients to control aggression,[2] enuresis,[3] fear, and minor social maladjustment in children.[2] re Its anxiolytic effects are most similar to diazepam, another anxiolytic, but unlike diazepam, benzoctamine has antagonistic effects on epinephrine and norepinephrine, and appears to increase serotonin levels. While little is understood about how it carries out its effects, studies point to reduced serotonin, epinephrine, and norepinephrine as partial causes of its pharmacologic and behavioral effects.
Animal studies have shown sedative hypnotic drugs tend to show dependency in animals, but benzoctamine has been shown to not be addictive. Other animal studies also point to the drug as a possible mechanism by which to reduce blood pressure through the adrenergic system.
Chemically, benzoctamine belongs to the class of compounds called dibenzobicyclo-octadienes. It is a tetracyclic compound, consisting of four rings in a three dimensional configuration, and is very closely related structurally to the tetracyclic antidepressant (TeCA) maprotiline, differing only in the length of their side chain.
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ a b Rickels K, Downing RW, Case WG, Feldman HS, Pereira-Ogan JA, Weise CC (March 1973). "Benzoctamine and Chlordiazepoxide in Anxiety: Some Predictors of Improvement". Journal of International Medical Research. 1 (3): 184–187. doi:10.1177/030006057300100308. ISSN 0300-0605.
- ^ Robson WL (2022-05-19). Cendron M (ed.). "Enuresis Treatment & Management: Approach Considerations, Initial Management, Alarm Therapy". MedScape.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search