
Back بتولینیک اسید AZB Бетулинова киселина Bulgarian Kyselina betulinová Czech Betulinsäure German Ácido betulínico Spanish بتولینیک اسید Persian Betuliinihappo Finnish Acido betulinico Italian Betulinezuur Dutch Acid betulinic Romanian
| |
| Names | |
|---|---|
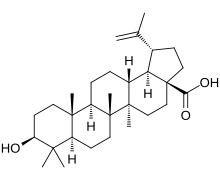
| IUPAC name
3β-Hydroxylup-20(29)-en-28-oic acid
| |
| Systematic IUPAC name
(1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-9-Hydroxy-5a,5b,8,8,11a-pentamethyl-1-(prop-1-en-1-yl)icosahydro-3aH-cyclopenta[a]chrysene-3a-carboxylic acid | |
| Other names
Betulic acid
Mairin | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.773 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C30H48O3 | |
| Molar mass | 456.711 g·mol−1 |
| Melting point | 316 to 318 °C (601 to 604 °F; 589 to 591 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Betulinic acid is a naturally occurring pentacyclic triterpenoid which has antiretroviral, antimalarial, and anti-inflammatory properties, as well as a more recently discovered potential as an anticancer agent, by inhibition of topoisomerase.[1] It is found in the bark of several species of plants, principally the white birch (Betula pubescens)[2] from which it gets its name, but also the ber tree (Ziziphus mauritiana), selfheal (Prunella vulgaris), the tropical carnivorous plants Triphyophyllum peltatum and Ancistrocladus heyneanus, Diospyros leucomelas, a member of the persimmon family, Tetracera boiviniana, the jambul (Syzygium formosanum),[3] flowering quince (Pseudocydonia sinensis, former Chaenomeles sinensis KOEHNE),[4] rosemary,[5] and Pulsatilla chinensis.[6]
- ^ Chowdhury AR, Mandal S, Mittra B, Sharma S, Mukhopadhyay S, Majumder HK (July 2002). "Betulinic acid, a potent inhibitor of eukaryotic topoisomerase I: identification of the inhibitory step, the major functional group responsible and development of more potent derivatives". Medical Science Monitor. 8 (7): BR254–65. PMID 12118187.
- ^ Tan Y, Yu R, Pezzuto JM (July 2003). "Betulinic acid-induced programmed cell death in human melanoma cells involves mitogen-activated protein kinase activation". Clinical Cancer Research. 9 (7): 2866–75. PMID 12855667.
- ^ Zuco V, Supino R, Righetti SC, Cleris L, Marchesi E, Gambacorti-Passerini C, Formelli F (January 2002). "Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells". Cancer Letters. 175 (1): 17–25. doi:10.1016/S0304-3835(01)00718-2. PMID 11734332.
- ^ Gao H, Wu L, Kuroyanagi M, Harada K, Kawahara N, Nakane T, Umehara K, Hirasawa A, Nakamura Y (November 2003). "Antitumor-promoting constituents from Chaenomeles sinensis KOEHNE and their activities in JB6 mouse epidermal cells". Chemical & Pharmaceutical Bulletin. 51 (11): 1318–21. doi:10.1248/cpb.51.1318. PMID 14600382. (Chaenomeles sinensis KOEHNE is now named Pseudocydonia sinensis)
- ^ Abe F, Yamauchi T, Nagao T, Kinjo J, Okabe H, Higo H, Akahane H (November 2002). "Ursolic acid as a trypanocidal constituent in rosemary". Biological & Pharmaceutical Bulletin. 25 (11): 1485–7. doi:10.1248/bpb.25.1485. PMID 12419966.
- ^ Ji ZN, Ye WC, Liu GG, Hsiao WL (November 2002). "23-Hydroxybetulinic acid-mediated apoptosis is accompanied by decreases in bcl-2 expression and telomerase activity in HL-60 Cells". Life Sciences. 72 (1): 1–9. doi:10.1016/S0024-3205(02)02176-8. PMID 12409140.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search