
Back بومبيكول Arabic Bombykol German Bombicol Spanish Bombükool Estonian Bombykoli Finnish Bombykol French Bombykol Hungarian Bombykol Italian ボンビコール Japanese Bombikol Polish

| |
| Names | |
|---|---|
| Preferred IUPAC name
(10E,12Z)-Hexadeca-10,12-dien-1-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H30O | |
| Molar mass | 238.415 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
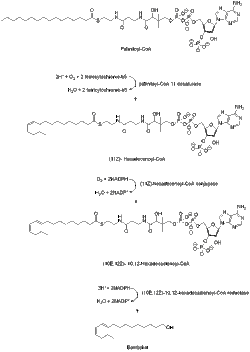
Bombykol is a pheromone released by the female silkworm moth to attract mates. It is also the sex pheromone in the wild silk moth (Bombyx mandarina).[1][2] Discovered by Adolf Butenandt in 1959, it was the first pheromone to be characterized chemically.[3]
Minute quantities of this pheromone can be used per acre of land to confuse male insects about the location of their female partners. It can thus serve as a lure in traps to remove insects effectively without spraying crops with large amounts of pesticides. Butenandt named the substance after the moth's Latin name Bombyx mori.[4]
In vivo it appears that bombykol is the natural ligand for a pheromone binding protein, BmorPBP, which escorts the pheromone to the pheromone receptor.[5]
- ^ Kuwahara, Yasumasa (1984). "Flight Time of Bombyx mandarina Males to a Pheromone Trap Baited with Bombykol". Applied Entomology and Zoology. 19 (3): 400–401. Bibcode:1984AppEZ..19..400K. doi:10.1303/aez.19.400.
- ^ Kuwahara, Yasumasa; Mori, Naoki; Yamada, Shigeki; Nemoto, Tadashi (1984). "Evaluation of Bombykol as the Sex Pheromone of Bombyx mandarina(Lepidoptera : Bombycidae)". Applied Entomology and Zoology. 19 (2): 265–267. Bibcode:1984AppEZ..19..265K. doi:10.1303/aez.19.265.
- ^ Butenandt, A.; Beckamnn, R.; Hecker, E. (1961). "Über den Sexuallockstoff des Seidenspinners .1. Der biologische Test und die Isolierung des reinen Sexuallockstoffes Bombykol". Hoppe-Seyler's Zeitschrift für Physiologische Chemie. 324: 71–83. doi:10.1515/bchm2.1961.324.1.71. PMID 13689417.
- ^ "Molecule of the Week". American Chemical Society. 2004. Archived from the original on August 7, 2004. Retrieved March 2, 2013.
- ^ Leal, Walter S. (2005). "Pheromone Reception". In Schulz, Stefan (ed.). The Chemistry of Pheromones and Other Semiochemicals II. Topics in Current Chemistry. Vol. 240. Springer. pp. 1–36. doi:10.1007/b98314. ISBN 9783540213086. Retrieved March 2, 2013.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search