
Back بوتورفانول Arabic Bwtorffanol Welsh Butorphanol German بوتورفانول Persian Butorfanoli Finnish Butorphanol French Butorfanolo Italian ブトルファノール Japanese Butorfanol Dutch ବୁଟୋରଫାନୋଲ OR
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Stadol, others |
| Other names | BC 2627 |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a682667 |
| Pregnancy category |
|
| Routes of administration | IV, intranasal, oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Nasal: 60-70%, Sublingual/Buccal 25%-35%. PO < OR= to 10%. * Try to use IN route best used out of the hospital setting or both choices. has longer duration of action ~ 5 hours. IV 2-4 hours. Avoid oral route unless stronger advised by a physician. |
| Metabolism | Liver hydroxylated & glucuronidated |
| Elimination half-life | 4-7 hours |
| Excretion | Kidney, 75% Biliary, 11-14% Fecal, 15% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.050.717 |
| Chemical and physical data | |
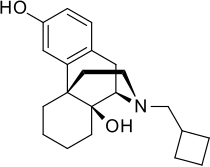
| Formula | C21H29NO2 |
| Molar mass | 327.468 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Butorphanol is a morphinan-type synthetic agonist–antagonist opioid analgesic developed by Bristol-Myers.[2][3][4][5][6] Butorphanol is most closely structurally related to levorphanol. Butorphanol is available as the tartrate salt in injectable, tablet, and intranasal spray formulations. The tablet form is only used in dogs, cats and horses due to low bioavailability in humans.
It was patented in 1971 and approved for medical use in 1979.[7]
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ US 3819635, "14-hydroxymorphinan derivatives."
- ^ US 3775414, "Process for the preparation of 14-hydroxymorphinan derivatives."
- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 200–. ISBN 978-1-4757-2085-3.
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 154–. ISBN 978-3-88763-075-1.
- ^ Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 58–. ISBN 978-94-011-4439-1.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 529. ISBN 978-3-527-60749-5.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search