Back ناقلة ميثيل-O الكاتيكول Arabic COMT BS COMT Welsh Catechol-O-Methyltransferase German Catecol O-metiltransferasa Spanish Katekoli-O-metyylitransferaasi Finnish Catechol-O-methyltransferase HE Katehol-o-metiltransferaza Croatian Catecolo O-metiltransferasi Italian カテコール-O-メチルトランスフェラーゼ Japanese
| catechol-O-methyltransferase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 2.1.1.6 | ||||||||
| CAS no. | 9012-25-3 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||

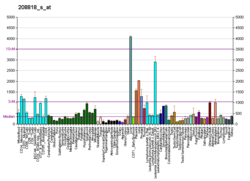
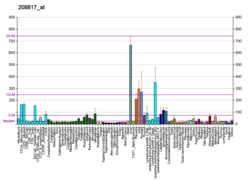
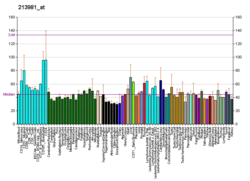
Catechol-O-methyltransferase (COMT; EC 2.1.1.6) is one of several enzymes that degrade catecholamines (neurotransmitters such as dopamine, epinephrine, and norepinephrine), catecholestrogens, and various drugs and substances having a catechol structure.[7] In humans, catechol-O-methyltransferase protein is encoded by the COMT gene.[8] Two isoforms of COMT are produced: the soluble short form (S-COMT) and the membrane bound long form (MB-COMT). As the regulation of catecholamines is impaired in a number of medical conditions, several pharmaceutical drugs target COMT to alter its activity and therefore the availability of catecholamines.[9] COMT was first discovered by the biochemist Julius Axelrod in 1957.[10]
- ^ a b c GRCh38: Ensembl release 89: ENSG00000093010 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000000326 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Flower R, Rang HP, Dale MM, Ritter JM (2007). "Figure 11-4". Rang & Dale's pharmacology (6th ed.). Edinburgh: Churchill Livingstone. ISBN 978-0-443-06911-6.
- ^ Rang HP, Dale MM, Ritter JM, Flower RJ, Henderson G (2011). "Figure 14.4". Rang & Dale's Pharmacology. Student consult (7th ed.). Elsevier Health Sciences. ISBN 978-0-7020-4504-2.
- ^ "Test ID: COMT: Catechol-O-Methyltransferase Genotype". mayomedicallaboratories.com. Mayo Clinic: Mayo Medical Laboratories. Archived from the original on September 18, 2008. Retrieved November 16, 2016.
- ^ Grossman MH, Emanuel BS, Budarf ML (April 1992). "Chromosomal mapping of the human catechol-O-methyltransferase gene to 22q11.1----q11.2". Genomics. 12 (4): 822–825. doi:10.1016/0888-7543(92)90316-K. PMID 1572656.
- ^ Tai CH, Wu RM (February 2002). "catechol-O-methyltransferase and Parkinson's disease". Acta Medica Okayama. 56 (1): 1–6. doi:10.18926/AMO/31725. PMID 11873938.
- ^ Axelrod J (August 1957). "O-methylation of epinephrine and other catechols in vitro and in vivo". Science. 126 (3270): 400–401. Bibcode:1957Sci...126..400A. doi:10.1126/science.126.3270.400. PMID 13467217.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search