
Back نظام مترافق Arabic Konjugirani sistem BS Sistema conjugat Catalan Konjugovaný systém Czech Konjugation (Chemie) German Sistema conjugado Spanish Konjugeerunud sidemed Estonian سیستم مزدوج Persian Konjugaatio (kemia) Finnish Système conjugué French


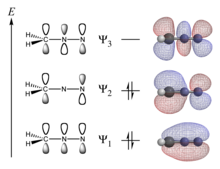
In theoretical chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally represented as having alternating single and multiple bonds. Lone pairs, radicals or carbenium ions may be part of the system, which may be cyclic, acyclic, linear or mixed. The term "conjugated" was coined in 1899 by the German chemist Johannes Thiele.[1]
Conjugation is the overlap of one p-orbital with another across an adjacent σ bond (in transition metals, d-orbitals can be involved).[2][a]
A conjugated system has a region of overlapping p-orbitals, bridging the interjacent locations that simple diagrams illustrate as not having a π bond. They allow a delocalization of π electrons across all the adjacent aligned p-orbitals.[3] The π electrons do not belong to a single bond or atom, but rather to a group of atoms.
Molecules containing conjugated systems of orbitals and electrons are called conjugated molecules, which have overlapping p orbitals on three or more atoms. Some simple organic conjugated molecules are 1,3-butadiene, benzene, and allylic carbocations.[4] The largest conjugated systems are found in graphene, graphite, conductive polymers and carbon nanotubes.
- ^ Thiele, Johannes (1899). "Zur Kenntnis der ungesättigten Verbindungen" [[Contribution] to our knowledge of unsaturated compounds]. Justus Liebig's Annalen der Chemie (in German). 306: 87–142. doi:10.1002/jlac.18993060107. On p. 90, Thiele coined the term "conjugated": "Ein solches System benachbarter Doppelbindungen mit ausgeglichenen inneren Partialvalenzen sei als 'conjugirt' bezeichnet." (Such a system of adjacent double bonds with equalized inner partial valences shall be termed "conjugated".)
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "conjugated system (conjugation)". doi:10.1351/goldbook.C01267
- ^ March, Jerry (1985). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, Inc. ISBN 0-471-85472-7.
- ^ "16 Conjugation, Resonance, and Dienes". Organic Chemistry (PDF) (3rd ed.). Belonia, South Tripura, India: Iswar Chandra Vidyasagar College. Retrieved 19 April 2022.
Cite error: There are <ref group=lower-alpha> tags or {{efn}} templates on this page, but the references will not show without a {{reflist|group=lower-alpha}} template or {{notelist}} template (see the help page).
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search