
Back ديفلازاكورت Arabic Deflazacort German Deflazacort Spanish Deflazacort French Deflazacort Italian ଡିଫ୍ଲାଜାକର୍ଟ OR Deflazakort Polish Deflazacorte Portuguese Deflazakort Serbo-Croatian Deflazakort Serbian
 | |
| Clinical data | |
|---|---|
| Trade names | Emflaza, Calcort, others |
| AHFS/Drugs.com | Monograph |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 40% |
| Metabolism | By plasma esterases, to active metabolite |
| Elimination half-life | 1.1–1.9 hours (metabolite) |
| Excretion | Kidney (70%) and fecal (30%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.034.969 |
| Chemical and physical data | |
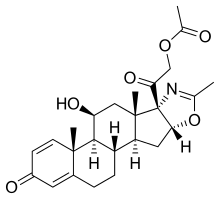
| Formula | C25H31NO6 |
| Molar mass | 441.524 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Deflazacort (trade name Calcort among others) is a glucocorticoid belonging to acetonides or O-isopropylidene derivative.[1] It is used as an anti-inflammatory and was patented in 1969[1] and approved for medical use in 1985.[2] The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication for Duchenne Muscular Dystrophy.[3]
- ^ a b Nayak S, Acharjya B (December 19, 2008). "Deflazacort versus other glucocorticoids: a comparison". Indian Journal of Dermatology. 53 (4): 167–170. doi:10.4103/0019-5154.44786. PMC 2763756. PMID 19882026.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 486. ISBN 9783527607495.
- ^ New Drug Therapy Approvals 2017 (PDF). U.S. Food and Drug Administration (FDA) (Report). January 2018. Retrieved 16 September 2020.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search