
Back دكسترين Arabic دکسترین AZB Дэкстрын Byelorussian Дэкстрын BE-X-OLD Декстрин Bulgarian ডেক্সট্রিন Bengali/Bangla Dekstrin BS Dextrina Catalan Dextrin Czech Dekstrin Danish

| |
| Identifiers | |
|---|---|
| ChemSpider |
|
| ECHA InfoCard | 100.029.693 |
| E number | E1400 (additional chemicals) |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| (C6H10O5)n | |
| Molar mass | variable |
| Appearance | white or yellow powder |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
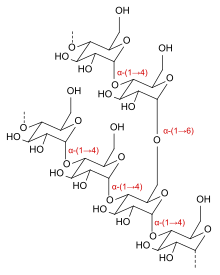
Dextrins are a group of low-molecular-weight carbohydrates produced by the hydrolysis of starch[1] and glycogen.[2] Dextrins are mixtures of polymers of D-glucose units linked by α-(1→4) or α-(1→6) glycosidic bonds.
Dextrins can be produced from starch using enzymes like amylases, as during digestion in the human body and during malting and mashing in beer brewing[3] or by applying dry heat under acidic conditions (pyrolysis or roasting). This procedure was first discovered in 1811 by Edme-Jean Baptiste Bouillon-Lagrange.[4] The latter process is used industrially, and also occurs on the surface of bread during the baking process, contributing to flavor, color and crispness. Dextrins produced by heat are also known as pyrodextrins. Starch hydrolyses during roasting under acidic conditions, and short-chained starch parts partially rebranch with α-(1,6) bonds to the degraded starch molecule.[5] See also Maillard reaction.
Dextrins are white, yellow, or brown powders that are partially or fully water-soluble, yielding optically active solutions of low viscosity. Most of them can be detected with iodine solution, giving a red coloration; one distinguishes erythrodextrin (dextrin that colours red) and achrodextrin (giving no colour).
White and yellow dextrins from starch roasted with little or no acid are called British gum.
- ^ An Introduction to the chemistry of plants - Vol II: Metabolic processes, P. Haas and T. G. Hill, London (Longmans, Green & Co.), 1913; pages 123-127
- ^ Salway, JG. Medical Biochemistry at a Glance. Second Edition. Malden, MA (Blackwell Publishing), 2006; page 66
- ^ Michael Lewis, Tom W. Young (2002), "Brewing", Kluwer Academic, ISBN 0-306-47274-0.
- ^ Edme-Jean-Baptiste Bouillon-Lagrange, Revista CENIC Ciencias Biológicas, Vol. 44, No. 1, mayo-agosto, 2013
- ^ Alistair M. Stephen, Glyn O. Phillips, Peter A. Williams (2006), "Food polysaccharides and their applications 2nd edition", p 92-99, CRC Press, Taylor & Francis Group, ISBN 0-8247-5922-2
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search