| |
| Names | |
|---|---|
| IUPAC name
Dicarbonate
| |
| Other names
Pyrocarbonate
| |
| Identifiers | |
3D model (JSmol)
|
|
| MeSH | pyrocarbonate |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2O2−5 | |
| Molar mass | 104.017 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |


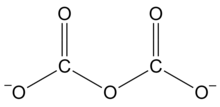
A dicarbonate, also known as a pyrocarbonate, is a chemical containing the divalent −O−C(=O)−O−C(=O)−O− or −C2O5− functional group, which consists of two carbonate groups sharing an oxygen atom. It is one of polycarbonate functional groups. These compounds can be viewed as derivatives of the hypothetical compound dicarbonic acid,[2] HO−C(=O)−O−C(=O)−OH or H2C2O5. Three important organic compounds containing this group are:
- dimethyl dicarbonate H3C−C2O5−CH3
- diethyl dicarbonate C2H5−C2O5−C2H5
- di-tert-butyl dicarbonate (H3C−)3C−C2O5−C(−CH3)3, also known as Boc anhydride.
It is one of the oxocarbon anions, consisting solely of oxygen and carbon. The anion has the formula −O−C(=O)−O−C(=O)−O− or C2O2−5. Dicarbonate salts are apparently unstable at ambient conditions, but can be made under pressure and may have a fleeting existence in carbonate solutions.[3]
The term dicarbonate is sometimes used erroneously to refer to bicarbonate, the common name of the hydrogencarbonate anion HCO−3 or esters of the hydrogencarbonate functional group −O−C(=O)−OH. It is also sometimes used for chemicals that contain two carbonate units in their covalent structure or stoichiometric formula.
- ^ Plácido García; Helge Willner; Maximiliano Burgos Paci; Gustavo A. Argüello; Thorsten Berends (2005). "Bis(trifluoromethyl)dicarbonate, CF3OC(O)OC(O)OCF3". J. Fluorine Chem. 126 (6): 984–990. doi:10.1016/j.jfluchem.2005.05.002.
- ^ "CHEBI:48501 - dicarbonic acid". ChEBI. Retrieved 30 June 2024.
- ^ Zeller, Klaus-Peter; Schuler, Paul; Haiss, Peter (2005). "The hidden equilibrium in aqueous sodium carbonate solutions: Evidence for the formation of the dicarbonate anion". Eur. J. Inorg. Chem. 2005 (1): 168–172. doi:10.1002/ejic.200400445.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search
