
Back إيكونازول Arabic Еконазол нитрат Bulgarian ইকোনাজোল Bengali/Bangla Econasol Welsh Econazol German Econazol Spanish اکونازول Persian Éconazole French इकोनाज़ोल Hindi Ekonazol Hungarian
 | |
| Clinical data | |
|---|---|
| Trade names | Spectazole, Ecostatin, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a684049 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.043.932 |
| Chemical and physical data | |
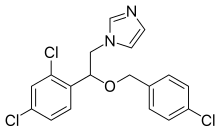
| Formula | C18H15Cl3N2O |
| Molar mass | 381.68 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Econazole is an antifungal medication of the imidazole class.[3]
It was patented in 1968, and approved for medical use in 1974.[4]
- ^ "Econazole topical Use During Pregnancy". Drugs.com. 3 September 2018. Retrieved 31 January 2020.
- ^ "Spectazole (econazole nitrate 1%) Cream". DailyMed. U.S. National Library of Medicine. 30 September 2013. Retrieved 31 July 2021.
- ^ Thienpont D, Van Cutsem J, Van Nueten JM, Niemegeers CJ, Marsboom R (February 1975). "Bilogical and toxicological properties of econazole, a broad-spectrum antimycotic". Arzneimittel-Forschung. 25 (2): 224–230. PMID 1173036.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 502. ISBN 9783527607495.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search