
Back انتقال الإلكترون Arabic Transfer elektrona BS Transferència electrònica Catalan Přenos elektronu Czech Elektronentransfer German Transferencia electrónica (química) Spanish انتقال الکترون Persian Transfer elektron ID Trasferimento di elettroni Italian 電子移動反応 Japanese
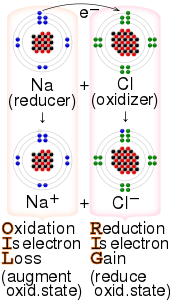
Electron transfer (ET) occurs when an electron relocates from an atom or molecule to another such chemical entity. ET is a mechanistic description of certain kinds of redox reactions involving transfer of electrons.[2]
Electrochemical processes are ET reactions. ET reactions are relevant to photosynthesis and respiration and commonly involve transition metal complexes.[3][4] In organic chemistry ET is a step in some commercial polymerization reactions. It is foundational to photoredox catalysis.
- ^ "Metals". Bitesize. BBC. Archived from the original on 2022-11-03.
- ^ Piechota, Eric J.; Meyer, Gerald J. (2019). "Introduction to Electron Transfer: Theoretical Foundations and Pedagogical Examples". Journal of Chemical Education. 96 (11): 2450–2466. Bibcode:2019JChEd..96.2450P. doi:10.1021/acs.jchemed.9b00489. S2CID 208754569.
- ^ Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 0-7506-3365-4.
- ^ Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search