
Back مستقبل مقترن بالبروتين ج Arabic Receptori spregnuti sa G-proteinom BS Receptor acoblat a proteïnes G Catalan Receptor spřažený s G proteinem Czech G-protein-koblede receptorer Danish G-Protein-gekoppelte Rezeptoren German Υποδοχέας συζευγμένος με πρωτεΐνη G Greek Receptor acoplado a proteínas G Spanish G-valguga seotud retseptorid Estonian G proteinei loturiko hartzaile Basque
| GPCR | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| Symbol | 7tm_1 | ||||||||
| Pfam | PF00001 | ||||||||
| InterPro | IPR000276 | ||||||||
| PROSITE | PDOC00210 | ||||||||
| TCDB | 9.A.14 | ||||||||
| OPM superfamily | 6 | ||||||||
| OPM protein | 1gzm | ||||||||
| CDD | cd14964 | ||||||||
| |||||||||
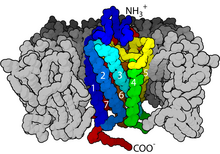
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. They are coupled with G proteins. They pass through the cell membrane seven times in the form of six loops[2] (three extracellular loops interacting with ligand molecules, three intracellular loops interacting with G proteins, an N-terminal extracellular region and a C-terminal intracellular region[2]) of amino acid residues, which is why they are sometimes referred to as seven-transmembrane receptors.[3] Ligands can bind either to the extracellular N-terminus and loops (e.g. glutamate receptors) or to the binding site within transmembrane helices (rhodopsin-like family). They are all activated by agonists, although a spontaneous auto-activation of an empty receptor has also been observed.[3]
G protein-coupled receptors are found only in eukaryotes, including yeast, and choanoflagellates.[4] The ligands that bind and activate these receptors include light-sensitive compounds, odors, pheromones, hormones, and neurotransmitters, and vary in size from small molecules to peptides to large proteins. G protein-coupled receptors are involved in many diseases.
There are two principal signal transduction pathways involving the G protein-coupled receptors:
- the cAMP signal pathway and
- the phosphatidylinositol signal pathway.[5]
When a ligand binds to the GPCR it causes a conformational change in the GPCR, which allows it to act as a guanine nucleotide exchange factor (GEF). The GPCR can then activate an associated G protein by exchanging the GDP bound to the G protein for a GTP. The G protein's α subunit, together with the bound GTP, can then dissociate from the β and γ subunits to further affect intracellular signaling proteins or target functional proteins directly depending on the α subunit type (Gαs, Gαi/o, Gαq/11, Gα12/13).[6]: 1160
GPCRs are an important drug target and approximately 34%[7] of all Food and Drug Administration (FDA) approved drugs target 108 members of this family. The global sales volume for these drugs is estimated to be 180 billion US dollars as of 2018[update].[7] It is estimated that GPCRs are targets for about 50% of drugs currently on the market, mainly due to their involvement in signaling pathways related to many diseases i.e. mental, metabolic including endocrinological disorders, immunological including viral infections, cardiovascular, inflammatory, senses disorders, and cancer. The long ago discovered association between GPCRs and many endogenous and exogenous substances, resulting in e.g. analgesia, is another dynamically developing field of the pharmaceutical research.[3]
- ^ Cite error: The named reference
Cherezov_2007was invoked but never defined (see the help page). - ^ a b Zhang, Jian V.; Li, Lei; Huang, Qingsheng; Ren, Pei-Gen (1 January 2013). "Chapter Three - Obestatin Receptor in Energy Homeostasis and Obesity Pathogenesis". In Tao, Ya-Xiong (ed.). Progress in Molecular Biology and Translational Science. G Protein-Coupled Receptors in Energy Homeostasis and Obesity Pathogenesis. Vol. 114. Academic Press. pp. 89–107. doi:10.1016/B978-0-12-386933-3.00003-0. ISBN 9780123869333. PMID 23317783. Archived from the original on 17 January 2023. Retrieved 24 October 2023.
- ^ a b c Trzaskowski B, Latek D, Yuan S, Ghoshdastider U, Debinski A, Filipek S (2012). "Action of molecular switches in GPCRs--theoretical and experimental studies". Current Medicinal Chemistry. 19 (8): 1090–109. doi:10.2174/092986712799320556. PMC 3343417. PMID 22300046.
 Text was copied from this source, which is available under a Attribution 2.5 Generic (CC BY 2.5) licence
Text was copied from this source, which is available under a Attribution 2.5 Generic (CC BY 2.5) licence
- ^ King N, Hittinger CT, Carroll SB (July 2003). "Evolution of key cell signaling and adhesion protein families predates animal origins". Science. 301 (5631): 361–3. Bibcode:2003Sci...301..361K. doi:10.1126/science.1083853. PMID 12869759. S2CID 9708224.
- ^ Gilman AG (1987). "G proteins: transducers of receptor-generated signals". Annual Review of Biochemistry. 56 (1): 615–49. doi:10.1146/annurev.bi.56.070187.003151. PMID 3113327.
- ^ Wettschureck N, Offermanns S (October 2005). "Mammalian G proteins and their cell type specific functions". Physiological Reviews. 85 (4): 1159–204. doi:10.1152/physrev.00003.2005. PMID 16183910.
- ^ a b Hauser AS, Chavali S, Masuho I, Jahn LJ, Martemyanov KA, Gloriam DE, Babu MM (January 2018). "Pharmacogenomics of GPCR Drug Targets". Cell. 172 (1–2): 41–54.e19. doi:10.1016/j.cell.2017.11.033. PMC 5766829. PMID 29249361.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search