
Back Hidroksied Afrikaans هيدروكسيد Arabic Hidróxidu AST هیدروکسید AZB Гідраксіды Byelorussian Хидроксид Bulgarian Hidroksid BS Hidròxid Catalan Hydroxidy Czech Hydroxid Danish
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Hydroxide
| |||
| Systematic IUPAC name
Oxidanide (not recommended) | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
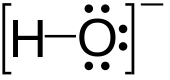
| OH− | |||
| Molar mass | 17.007 g·mol−1 | ||
| Conjugate acid | Water | ||
| Conjugate base | Oxide anion | ||
| Related compounds | |||
Related compounds
|
O2H+ OH• O22− H2O | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry.
Many inorganic substances which bear the word hydroxide in their names are not ionic compounds of the hydroxide ion, but covalent compounds which contain hydroxy groups.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search

