Back إيكاريدين Arabic ایکاریدین AZB Icaridin German Icaridina Spanish ایکاریدین Persian Ikaridiini Finnish Icaridine French Icaridina Italian イカリジン Japanese Икаридин Macedonian
| |||
| Names | |||
|---|---|---|---|
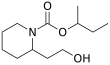
| Preferred IUPAC name
Butan-2-yl 2-(2-hydroxyethyl)piperidine-1-carboxylate | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.102.177 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C12H23NO3 | |||
| Molar mass | 229.320 g·mol−1 | ||
| Appearance | colorless liquid | ||
| Odor | odorless | ||
| Density | 1.07 g/cm3 | ||
| Melting point | −170 °C (−274 °F; 103 K) | ||
| Boiling point | 296 °C (565 °F; 569 K) | ||
| 0.82 g/100 mL | |||
| Solubility | 752 g/100mL (acetone) | ||
Refractive index (nD)
|
1.4717 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Icaridin, also known as picaridin, is an insect repellent which can be used directly on skin or clothing.[1] It has broad efficacy against various arthropods such as mosquitos, ticks, gnats, flies and fleas, and is almost colorless and odorless. A study performed in 2010 showed that picaridin spray and cream at the 20% concentration provided 12 hours of protection against ticks.[2] Unlike DEET, icaridin does not dissolve plastics, synthetics or sealants,[3] is odorless and non-greasy[4] and presents a lower risk of toxicity when used with sunscreen, as it may reduce skin absorption of both compounds.[5]
The name picaridin was proposed as an International Nonproprietary Name (INN) to the World Health Organization (WHO), but the official name that has been approved by the WHO is icaridin. The chemical is part of the piperidine family,[1] along with many pharmaceuticals and alkaloids such as piperine, which gives black pepper its spicy taste.
Trade names include Bayrepel and Saltidin among others. The compound was developed by the German chemical company Bayer in the 1980s [6] and was given the name Bayrepel. In 2005, Lanxess AG and its subsidiary Saltigo GmbH were spun off from Bayer[7] and the product was renamed Saltidin in 2008.[8]
Having been sold in Europe since 1998,[9] on 23 July 2020, icaridin was approved again by the EU Commission for use in repellent products. The approval entered into force on 1 February 2022 and is valid for ten years.[10]
- ^ a b "Picaridin". npic.orst.edu. Retrieved 2020-03-29.
- ^ https://archive.epa.gov/hsrb/web/pdf/2a_lnx003_primary_report_mrid_480538011.pdf [bare URL PDF]
- ^ Picaridin. Archived from the original on August 9, 2011.
- ^ "Picaridin vs DEET: Which Is the Best Insect Repellent?". Appalachian Mountain Club. 4 August 2023. Retrieved 7 August 2023.
- ^ Rodriguez J, Maibach HI (2016-01-02). "Percutaneous penetration and pharmacodynamics: Wash-in and wash-off of sunscreen and insect repellent". Journal of Dermatological Treatment. 27 (1): 11–18. doi:10.3109/09546634.2015.1050350. ISSN 0954-6634. PMID 26811157. S2CID 40319483.
- ^ "Picaridin Technical Fact Sheet". National Pesticide information Center.
- ^ "Bayer Completes Spin Off of Lanxess AG". 31 January 2005.
- ^ Saltigo renames insect repellant, Chemical & Engineering News
- ^ "Icaridin - an overview". ScienceDirect. Retrieved 29 December 2023.
- ^ "Commission Implementing Regulation (EU) 2020/1086 of 23 July 2020 approving icaridin as an existing active substance for use in biocidal products of product-type 19". Retrieved 31 August 2020.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search