
Back إندان Arabic ایندان AZB Indan Czech Indan German Indano Esperanto ایندان Persian Indane French Ինդան Armenian インダン Japanese Indaan Dutch

| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,3-Dihydro-1H-indene[2] | |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| 1904376 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.105 |
| 67817 | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H10 | |
| Molar mass | 118.176 g/mol |
| Appearance | colorless liquid |
| Density | 0.9645 g/cm3 |
| Melting point | −51.4 °C (−60.5 °F; 221.8 K) |
| Boiling point | 176.5 °C (349.7 °F; 449.6 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
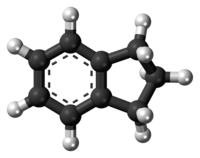
Indane or indan is an organic compound with the formula C6H4(CH2)3. It is a colorless liquid hydrocarbon. It is a petrochemical, a bicyclic compound. It occurs at the level of about 0.1% in coal tar. It is usually produced by hydrogenation of indene.[3]
- ^ a b Hawley, Gessner G. (1977). The Condensed Chemical Dictionary. Van Nostrand Reinhold Company. p. 464. ISBN 0-442-23240-3.
- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 602. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ Karl Griesbaum, Arno Behr, Dieter Biedenkapp, Heinz-Werner Voges, Dorothea Garbe, Christian Paetz, Gerd Collin, Dieter Mayer, Hartmut Höke "Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry 2002 Wiley-VCH, Weinheim. doi:10.1002/14356007.a13_227
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search