
Back تخمر لبني Arabic Млечнокисела ферментация Bulgarian Fermentació làctica Catalan Milchsäuregärung German Fermentación láctica Spanish Hartzidura laktiko Basque Maitohappokäyminen Finnish Fermentation lactique French Fermentación láctica Galician תסיסה הומולקטית HE
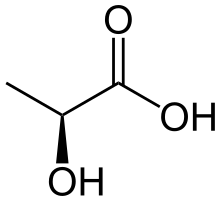
Lactic acid fermentation is a metabolic process by which glucose or other six-carbon sugars (also, disaccharides of six-carbon sugars, e.g. sucrose or lactose) are converted into cellular energy and the metabolite lactate, which is lactic acid in solution. It is an anaerobic fermentation reaction that occurs in some bacteria and animal cells, such as muscle cells.[1][2][3][page needed]
If oxygen is present in the cell, many organisms will bypass fermentation and undergo cellular respiration; however, facultative anaerobic organisms will both ferment and undergo respiration in the presence of oxygen.[3] Sometimes even when oxygen is present and aerobic metabolism is happening in the mitochondria, if pyruvate is building up faster than it can be metabolized, the fermentation will happen anyway.
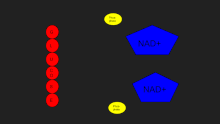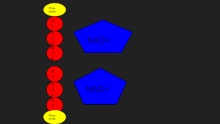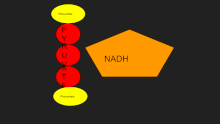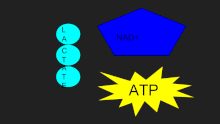
Lactate dehydrogenase catalyzes the interconversion of pyruvate and lactate with concomitant interconversion of NADH and NAD+.
In homolactic fermentation, one molecule of glucose is ultimately converted to two molecules of lactic acid. Heterolactic fermentation, by contrast, yields carbon dioxide and ethanol in addition to lactic acid, in a process called the phosphoketolase pathway.[1]
- ^ a b Battcock M, Azam-Ali S (1998). "Bacterial Fermentations". Fermented Fruits and Vegetables: A Global Perspective. Food and Agriculture Organization of the United Nations. ISBN 92-5-104226-8. Archived from the original on 2019-02-24. Retrieved 2007-06-10.
- ^ Abedon ST (1998-04-03). "Glycolysis and Fermentation". Ohio State University. Archived from the original on 2010-01-17. Retrieved 2010-01-12.
- ^ a b Campbell N, Reece J (2005). Biology (7th ed.). Benjamin Cummings. ISBN 0-8053-7146-X.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search