
Back Lamivudien Afrikaans لاميفودين Arabic Lamifwdin Welsh Lamivudin German Lamivudina Spanish Lamivudiin Estonian Lamibudina Basque لامیوودین Persian Lamivudiini Finnish Lamivudine French
 | |
| Clinical data | |
|---|---|
| Trade names | Epivir, Epivir-HBV, Zeffix, others[1] |
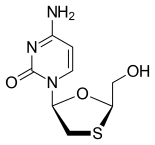
| Other names | (−)-L-2′,3′-dideoxy-3′-thiacytidine |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a696011 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 86% |
| Protein binding | Less than 36% |
| Elimination half-life | 5 to 7 hours |
| Excretion | Kidney (circa 70%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.132.250 |
| Chemical and physical data | |
| Formula | C8H11N3O3S |
| Molar mass | 229.25 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Lamivudine, commonly called 3TC, is an antiretroviral medication used to prevent and treat HIV/AIDS.[1] It is also used to treat chronic hepatitis B when other options are not possible.[1] It is effective against both HIV-1 and HIV-2.[1] It is typically used in combination with other antiretrovirals such as zidovudine, dolutegravir, and abacavir.[1] Lamivudine may be included as part of post-exposure prevention in those who have been potentially exposed to HIV.[1] Lamivudine is taken by mouth as a liquid or tablet.[1]
Common side effects include nausea, diarrhea, headaches, feeling tired, and cough.[1] Serious side effects include liver disease, lactic acidosis, and worsening hepatitis B among those already infected.[1] It is safe for people over three months of age and can be used during pregnancy.[1] The medication can be taken with or without food.[1] Lamivudine is a nucleoside reverse transcriptase inhibitor and works by blocking the HIV reverse transcriptase and hepatitis B virus polymerase.[1]
Lamivudine was patented in 1995 and approved for use in the United States in 1995.[8][9] It is on the World Health Organization's List of Essential Medicines.[10] It is available as a generic medication.[1]
- ^ a b c d e f g h i j k l m "Lamivudine". The American Society of Health-System Pharmacists. Archived from the original on 2 June 2016. Retrieved 31 July 2016.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "3TC (lamivudine, Epivir)". Catie. 2014. Retrieved 22 August 2022.
- ^ "Epivir- lamivudine tablet, film coated Epivir- lamivudine solution". DailyMed. 1 August 2020. Retrieved 28 November 2020.
- ^ "Epivir HBV- lamivudine tablet, film coated Epivir HBV- lamivudine solution". DailyMed. 17 August 2020. Retrieved 28 November 2020.
- ^ Cite error: The named reference
Epivir EPARwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Zeffix EPARwas invoked but never defined (see the help page). - ^ Therapy of Viral Infections Volume 15 of Topics in Medicinal Chemistry. Springer. 2015. p. 6. ISBN 9783662467596. Archived from the original on 15 August 2016.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 506. ISBN 9783527607495.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search