
Back ليدوكائين Arabic Lidokain Azerbaijani لیدوکین AZB Лидокаин Bulgarian লিডোকেইন Bengali/Bangla Lidocaïna Catalan لیدۆکاین CKB Lidokain Czech Lidocain Welsh Lidokain Danish
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | Lidocaine /ˈlaɪdəˌkeɪn/[1][2] Lignocaine /ˈlɪɡnəˌkeɪn/ |
| Trade names | Xylocaine, Ztlido, others |
| Other names | lignocaine |
| AHFS/Drugs.com | Local Monograph
Systemic Monograph Ophthalmic Professional Drug Facts |
| MedlinePlus | a682701 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intravenous, subcutaneous, topical, by mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 35% (by mouth) 3% (topical) |
| Metabolism | Liver,[7] 90% CYP3A4-mediated |
| Onset of action | Within 1.5 min (IV)[7] |
| Elimination half-life | 1.5 h to 2 h |
| Duration of action | 10 min to 20 min (IV),[7] 0.5 h to 3 h (local)[8][9] |
| Excretion | Kidney[7] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI |
|
| ChEMBL |
|
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.821 |
| Chemical and physical data | |
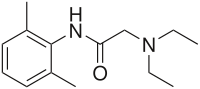
| Formula | C14H22N2O |
| Molar mass | 234.343 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 68 °C (154 °F) |
| |
| |
| (verify) | |
Lidocaine, also known as lignocaine and sold under the brand name Xylocaine among others, is a local anesthetic of the amino amide type. It is also used to treat ventricular tachycardia.[7][8] When used for local anaesthesia or in nerve blocks, lidocaine typically begins working within several minutes and lasts for half an hour to three hours.[8][9] Lidocaine mixtures may also be applied directly to the skin or mucous membranes to numb the area.[8] It is often used mixed with a small amount of adrenaline (epinephrine) to prolong its local effects and to decrease bleeding.[8]
If injected intravenously, it may cause cerebral effects such as confusion, changes in vision, numbness, tingling, and vomiting.[7] It can cause low blood pressure and an irregular heart rate.[7] There are concerns that injecting it into a joint can cause problems with the cartilage.[8] It appears to be generally safe for use in pregnancy.[7] A lower dose may be required in those with liver problems.[7] It is generally safe to use in those allergic to tetracaine or benzocaine.[8] Lidocaine is an antiarrhythmic medication of the class Ib type.[7] This means it works by blocking sodium channels and thus decreasing the rate of contractions of the heart.[7] When injected near nerves, the nerves cannot conduct signals to or from the brain.[8]
Lidocaine was discovered in 1946 and went on sale in 1948.[10] It is on the World Health Organization's List of Essential Medicines.[11] It is available as a generic medication.[8][12] In 2021, it was the 267th most commonly prescribed medication in the United States, with more than 1 million prescriptions.[13][14]
- ^ "Lidocaine". Merriam-Webster.com Dictionary.
- ^ "Lidocaine". Dictionary.com Unabridged (Online). n.d.
- ^ "Poisons Standard February 2021". Federal Register of Legislation. 1 January 2021. Retrieved 11 April 2021.
- ^ "Lidocaine Hydrochloride Injection BP 1% w/v - Summary of Product Characteristics (SmPC)". (emc). 29 June 2020. Retrieved 11 April 2021.
- ^ "Xylocaine MPF- lidocaine hydrochloride injection, solution Xylocaine- lidocaine hydrochloride injection, solution Xylocaine- lidocaine hydrochloride,epinephrine bitartrate injection, solution". DailyMed. Retrieved 11 April 2021.
- ^ "Ztlido- lidocaine patch". DailyMed. Retrieved 11 April 2021.
- ^ a b c d e f g h i j k "Lidocaine Hydrochloride (Antiarrhythmic)". The American Society of Health-System Pharmacists. Archived from the original on 10 August 2015. Retrieved 26 August 2015.
- ^ a b c d e f g h i "Lidocaine Hydrochloride (Local)". The American Society of Health-System Pharmacists. Archived from the original on 6 September 2015. Retrieved 26 August 2015.
- ^ a b Nolan JP, Baskett PJ (1997). "Analgesia and anaesthesia". In David Skinner, Andrew Swain, Rodney Peyton, Colin Robertson (eds.). Cambridge Textbook of Accident and Emergency Medicine. Project co-ordinator, Fiona Whinster. Cambridge, UK: Cambridge University Press. p. 194. ISBN 9780521433792. Archived from the original on 8 September 2017.
- ^ Scriabine A (1999). "Discovery and development of major drugs currently in use". In Ralph Landau, Basil Achilladelis, Alexander Scriabine (eds.). Pharmaceutical Innovation: Revolutionizing Human Health. Philadelphia: Chemical Heritage Press. p. 211. ISBN 9780941901215. Archived from the original on 8 September 2017.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ Hamilton R (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 22. ISBN 9781284057560.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Lidocaine - Drug Usage Statistics". ClinCalc. Retrieved 14 January 2024.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search