
Back كاشف محدد Arabic Reactivo limitante Spanish Erreaktibo mugatzaile Basque واکنشدهنده محدودساز Persian Réactif limitant French सीमान्त अभिकर्मक Hindi Reyaktif limitatif HT Pereaksi pembatas ID Reagente limitante Italian Reagen pengehad Malay
This article needs additional citations for verification. (June 2015) |
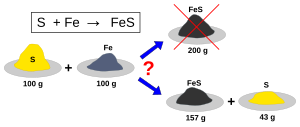
The limiting reagent (or limiting reactant or limiting agent) in a chemical reaction is a reactant that is totally consumed when the chemical reaction is completed.[1][2] The amount of product formed is limited by this reagent, since the reaction cannot continue without it. If one or more other reagents are present in excess of the quantities required to react with the limiting reagent, they are described as excess reagents or excess reactants (sometimes abbreviated as "xs"), or to be in abundance.[3]
The limiting reagent must be identified in order to calculate the percentage yield of a reaction since the theoretical yield is defined as the amount of product obtained when the limiting reagent reacts completely. Given the balanced chemical equation, which describes the reaction, there are several equivalent ways to identify the limiting reagent and evaluate the excess quantities of other reagents.
- ^ Olmsted, John; Williams, Gregory M. (1997). Chemistry: The Molecular Science. Jones & Bartlett Learning. p. 163. ISBN 0815184506.
- ^ Zumdahl, Steven S. (2006). Chemical Principles (4th ed.). New York: Houghton Mifflin Company. ISBN 0-618-37206-7.
- ^ Masterton, William L.; Hurley, Cecile N. (2008). Chemistry: Principles and Reactions (6 ed.). Cengage Learning. ISBN 978-0-495-12671-3.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search