
Back لوتيبريدنول Arabic Loteprednolo Italian ロテプレドノール Japanese ଲୋଟେପ୍ରେଡନୋଲ OR Loteprednol Portuguese Loteprednol Serbo-Croatian Loteprednol Serbian Loteprednol Vietnamese
 | |
| Clinical data | |
|---|---|
| Trade names | Lotemax |
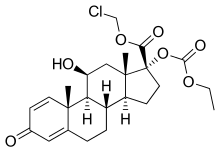
| Other names | 11β,17α,Dihydroxy-21-oxa-21-chloromethylpregna-1,4-diene-3,20-dione 17α-ethylcarbonate |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | Eye drops |
| Drug class | Corticosteroid; glucocorticoid |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | None |
| Protein binding | 95% |
| Metabolism | Ester hydrolysis |
| Metabolites | Δ1-cortienic acid and its etabonate |
| Onset of action | ≤2 hrs (allergic conjunctivitis) |
| Elimination half-life | 2.8 hrs |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.167.120 |
| Chemical and physical data | |
| Formula | C24H31ClO7 |
| Molar mass | 466.96 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 220.5 to 223.5 °C (428.9 to 434.3 °F) |
| Solubility in water | 0.0005 mg/mL (20 °C) |
| |
| |
| | |
Loteprednol (synthesized as the ester loteprednol etabonate) is a topical corticosteroid used to treat inflammations of the eye. It is marketed by Bausch and Lomb as Lotemax[2] and Loterex.
It was patented in 1980 and approved for medical use in 1998.[3] It is available as a generic medication.[4]
- ^ "Prescription medicines: registration of new chemical entities in Australia, 2014". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 10 April 2023.
- ^ Cite error: The named reference
ACwas invoked but never defined (see the help page). - ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 488. ISBN 9783527607495.
- ^ "First Generic Drug Approvals 2023". U.S. Food and Drug Administration (FDA). 30 May 2023. Archived from the original on 30 June 2023. Retrieved 30 June 2023.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search