
Back MBDB Czech MBDB German MBDB French MBDB Italian N-Metylo-1,3-benzodioksolilobutanoamina Polish MBDB Russian 1,3-Benzodioksolil-N-metilbutanamin Serbo-Croatian 1,3-Benzodioksolil-N-metilbutanamin Serbian
This article needs additional citations for verification. (November 2013) |
 | |
 Chemical structure | |
| Clinical data | |
|---|---|
| Other names | Methylbenzodioxolylbutanamine; N-Methyl-1,3-benzodioxolylbutanamine; MBDB; 3,4-Methylenedioxy-N-methyl-butanphenamine; MDMB |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C12H17NO2 |
| Molar mass | 207.273 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 156 °C (313 °F) |
| |
| |
| (verify) | |
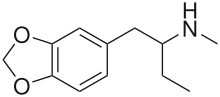
1,3-Benzodioxolyl-N-methylbutanamine (N-methyl-1,3-benzodioxolylbutanamine, MBDB, 3,4-methylenedioxy-N-methyl-α-ethylphenylethylamine) is an entactogen of the phenethylamine chemical class. It is known by the street names Eden and Methyl-J.[1] MBDB is a ring substituted amphetamine and an analogue of MDMA. Like MDMA, it has a methylene dioxy substitution at the 3 and 4 position on the aromatic ring; this is perhaps the most distinctive feature that structurally define analogues of MDMA, in addition to their unique effects, and as a class they are often referred to as "entactogens" to differentiate between typical psychostimulant amphetamines that (as a general rule) are not ring substituted. MBDB differs from MDMA by having an ethyl group instead of a methyl group attached to the alpha carbon; all other parts are identical. Modification at the alpha carbon is uncommon for substituted amphetamines. It has IC50 values of 784 nM against 5-HT, 7825 nM against dopamine, and 1233 nM against norepinephrine.[citation needed] Its metabolism has been described in scientific literature.[2]
MBDB was first synthesized by pharmacologist and medicinal chemist David E. Nichols[citation needed] and later tested by Alexander Shulgin and described in his book, PiHKAL: A Chemical Love Story. MBDB's dosage, according to PiHKAL, is 180–210 mg; the proper dosage relative to body mass seems unknown. Its duration is 4–6 hours, with noticeable after-effects lasting for 1–3 hours.
MBDB was initially developed as a non-psychedelic entactogen. It has lower effects on the dopamine system in comparison to other entactogens such as MDMA.[citation needed] MBDB causes many mild, MDMA-like effects, in particular the lowering of social barriers and inhibitions, pronounced sense of empathy and compassion, mood lift, and mild euphoria, all of which are present.[citation needed] MBDB tends to produce less euphoria, psychedelia, and stimulation in comparison to MDMA.[citation needed]
- ^ "MBDB". Erowid Center.
- ^ Lai FY, Erratico C, Kinyua J, Mueller JF, Covaci A, van Nuijs AL (October 2015). "Liquid chromatography-quadrupole time-of-flight mass spectrometry for screening in vitro drug metabolites in humans: investigation on seven phenethylamine-based designer drugs" (PDF). Journal of Pharmaceutical and Biomedical Analysis. 114: 355–75. doi:10.1016/j.jpba.2015.06.016. hdl:10067/1278220151162165141. PMID 26112925.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search