
Back ميلوكسيكام Arabic Meloxicam AST مئلوکسیکام AZB Meloksikam BS Melocsicam Welsh Meloxicam German Μελοξικάμη Greek Meloksikamo Esperanto Meloxicam Spanish ملوکسیکام Persian
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Mobic, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601242 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 89%[7] |
| Protein binding | 99.4%[7] |
| Metabolism | Liver (CYP2C9 and 3A4-mediated)[7] |
| Elimination half-life | 20 hours[7] |
| Excretion | Urine and feces equally[7] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.113.257 |
| Chemical and physical data | |
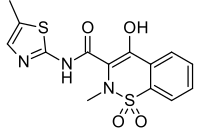
| Formula | C14H13N3O4S2 |
| Molar mass | 351.40 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Meloxicam, sold under the brand name Mobic among others, is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain and inflammation in rheumatic diseases and osteoarthritis.[8][9] It is used by mouth or by injection into a vein.[9][10] It is recommended that it be used for as short a period as possible and at a low dose.[9]
Common side effects include abdominal pain, dizziness, swelling, headache, and a rash.[9] Serious side effects may include heart disease, stroke, kidney problems, and stomach ulcers.[9] Use is not recommended in the third trimester of pregnancy.[9] It blocks cyclooxygenase-2 (COX-2) more than it blocks cyclooxygenase-1 (COX-1).[9] It is in the oxicam family of chemicals and is closely related to piroxicam.[9]
Meloxicam was patented in 1977 and approved for medical use in the United States in 2000.[9][11] It was developed by Boehringer Ingelheim; however, it is also available as a generic medication.[9] In 2021, it was the 32nd most commonly prescribed medication in the United States, with more than 18 million prescriptions.[12][13] An intravenous version of meloxicam (Anjeso) was approved for medical use in the United States in February 2020.[14][10]
- ^ Use During Pregnancy and Breastfeeding
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Health product highlights 2021: Annexes of products approved in 2021". Health Canada. 3 August 2022. Retrieved 25 March 2024.
- ^ "Mobic- meloxicam tablet". DailyMed. Retrieved 15 May 2021.
- ^ "Anjeso- meloxicam injection". DailyMed. Retrieved 15 May 2021.
- ^ "Loxitab EPAR". European Medicines Agency. 8 September 2023. Retrieved 24 May 2024.
- ^ a b c d e Cite error: The named reference
drugs1996was invoked but never defined (see the help page). - ^ British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 1112–1113. ISBN 9780857113382.
- ^ a b c d e f g h i j "Meloxicam Monograph for Professionals". Drugs.com. AHFS. Archived from the original on 23 December 2018. Retrieved 23 December 2018.
- ^ a b Cite error: The named reference
Anjeso PRwas invoked but never defined (see the help page). - ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 519. ISBN 9783527607495. Archived from the original on 10 July 2020. Retrieved 30 June 2020.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Meloxicam - Drug Usage Statistics". ClinCalc. Retrieved 14 January 2024.
- ^ "Anjeso- meloxicam injection". DailyMed. 22 February 2022. Retrieved 8 October 2022.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search