
Back متارامینول AZB Metaraminol Welsh Metaraminol German متارامینول Persian メタラミノール Japanese Metaraminol Portuguese Metaraminol Serbo-Croatian Metaraminol Serbian Metaraminol Vietnamese 间羟胺 Chinese
 | |
| Clinical data | |
|---|---|
| Trade names | Aramine, Metaramin, Pressonex |
| AHFS/Drugs.com | International Drug Names |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous, endotracheal |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | n/a |
| Protein binding | ~45% |
| Metabolism | Liver |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
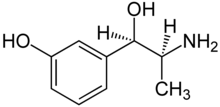
| Formula | C9H13NO2 |
| Molar mass | 167.208 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Metaraminol, previously sold under the brand name Aramine among others and also known as metaradrine, is a stereoisomer of meta-hydroxynorephedrine (3,β-dihydroxyamphetamine), is a potent sympathomimetic amine used in the prevention and treatment of hypotension, particularly as a complication of anesthesia. It is an α1-adrenergic receptor agonist with some β-adrenergic effect.[2] It is currently sold in its generic form by Slayback Pharma.[3]
- ^ "Injection : Aramine (Metaraminol Bitartrate)" (PDF). U.S. Food and Drug Administration (FDA). Retrieved 12 March 2022.
- ^ Kee VR (August 2003). "Hemodynamic pharmacology of intravenous vasopressors". Crit Care Nurse. 23 (4): 79–82. doi:10.4037/ccn2003.23.4.79. PMID 12961786.
- ^ "ANDA Approval for Metaraminol" (PDF). United States Food and Drug Administration. 24 August 2021. Retrieved 13 August 2022.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search