
Back متازوسین Persian Metazocin Croatian Metazocyna Polish Metazocin Serbo-Croatian Metazocin Serbian Metazocin Swedish
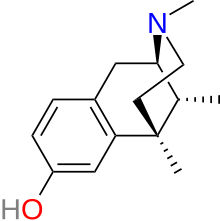 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.020.998 |
| Chemical and physical data | |
| Formula | C15H21NO |
| Molar mass | 231.339 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Metazocine is an opioid analgesic related to pentazocine. While metazocine has significant analgesic effects,[2] mediated through a mixed agonist–antagonist action[3] at the mu opioid receptor,[4] its clinical use is limited by dysphoric and hallucinogenic effects which are most likely caused by activity at kappa opioid receptors (where it is a high-efficacy agonist)[5] and/or sigma receptors.[6][7]
Metazocine is in Schedule II of the Controlled Substances Act 1970 of the United States as a Narcotic with ACSCN 9240 with a 19 gram aggregate manufacturing quota as of 2014. The free base conversion ratio for salts includes 0.81 for the hydrochloride and 0.74 for the hydrobromide.[8] It is listed under the Single Convention for the Control of Narcotic Substances 1961 and is controlled in most countries in the same fashion as is morphine.
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ Hori M, Ban M, Imai E, Iwata N, Suzuki Y, Baba Y, Morita T, Fujimura H, Nozaki M, Niwa M (November 1985). "Novel nonnarcotic analgesics with an improved therapeutic ratio. Structure-activity relationships of 8-(methylthio)- and 8-(acylthio)-1,2,3,4,5,6-hexahydro-2,6-methano-3-benzazocines". Journal of Medicinal Chemistry. 28 (11): 1656–61. doi:10.1021/jm00149a020. PMID 2999399.
- ^ Berzetei-Gurske I, Loew GH (1990). "The novel antagonist profile of (-)metazocine". Progress in Clinical and Biological Research. 328: 33–6. PMID 2154788.
- ^ Gharagozlou P, Demirci H, David Clark J, Lameh J (January 2003). "Activity of opioid ligands in cells expressing cloned mu opioid receptors". BMC Pharmacology. 3: 1. doi:10.1186/1471-2210-3-1. PMC 140036. PMID 12513698.
- ^ Gharagozlou P, Hashemi E, DeLorey TM, Clark JD, Lameh J (January 2006). "Pharmacological profiles of opioid ligands at kappa opioid receptors". BMC Pharmacology. 6: 3. doi:10.1186/1471-2210-6-3. PMC 1403760. PMID 16433932.
- ^ Shannon HE (July 1982). "Pharmacological analysis of the phencyclidine-like discriminative stimulus properties of narcotic derivatives in rats". The Journal of Pharmacology and Experimental Therapeutics. 222 (1): 146–51. PMID 7086696.
- ^ Slifer BL, Balster RL, May EL (October 1986). "Reinforcing and phencyclidine-like stimulus properties of enantiomers of metazocine". Pharmacology, Biochemistry, and Behavior. 25 (4): 785–9. doi:10.1016/0091-3057(86)90388-6. PMID 3786338. S2CID 32126170.
- ^ "Quotas - 2014". Diversion Control Division. U.S. Department of Justice, Drug Enforcement Administration.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search