
Back مترونيدازول Arabic Metronidazol AST مترونیدازول AZB মেট্রোনিডাজল Bengali/Bangla Metronidazole Catalan مێترۆنیدازۆل CKB Metronidasol Welsh Metronidazol Danish Metronidazol German Μετρονιδαζόλη Greek
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Flagyl |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a689011 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, topical, rectal, intravenous, vaginal |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 80% (by mouth), 60–80% (rectal), 20–25% (vaginal)[6][7][8] |
| Protein binding | 20%[6][7] |
| Metabolism | Liver[6][7] |
| Metabolites | Hydroxymetronidazole |
| Elimination half-life | 8 hours[6][7] |
| Excretion | Urine (77%), faeces (14%)[6][7] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.489 |
| Chemical and physical data | |
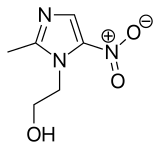
| Formula | C6H9N3O3 |
| Molar mass | 171.156 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 159 to 163 °C (318 to 325 °F) |
| |
| |
| (verify) | |
Metronidazole, sold under the brand name Flagyl among others, is an antibiotic and antiprotozoal medication.[9] It is used either alone or with other antibiotics to treat pelvic inflammatory disease, endocarditis, and bacterial vaginosis.[9] It is effective for dracunculiasis, giardiasis, trichomoniasis, and amebiasis.[9] It is an option for a first episode of mild-to-moderate Clostridioides difficile colitis if vancomycin or fidaxomicin is unavailable.[9][10] Metronidazole is available orally (by mouth), as a cream or gel, and by slow intravenous infusion (injection into a vein).[9][3]
Common side effects include nausea, a metallic taste, loss of appetite, and headaches.[9] Occasionally seizures or allergies to the medication may occur.[9] Some state that metronidazole should not be used in early pregnancy, while others state doses for trichomoniasis are safe.[1][weasel words] Metronidazole is generally considered compatible with breastfeeding.[1][11]
Metronidazole began to be commercially used in 1960 in France.[12] It is on the World Health Organization's List of Essential Medicines.[13] It is available in most areas of the world.[14] In 2021, it was the 194th most commonly prescribed medication in the United States, with more than 2 million prescriptions.[15][16]
- ^ a b c "Metronidazole Use During Pregnancy". www.drugs.com. Archived from the original on 1 January 2017. Retrieved 1 January 2017.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ a b "Metronidazole injection, solution". DailyMed. 16 January 2023. Archived from the original on 6 September 2023. Retrieved 5 July 2023.
- ^ "Metronidazole tablet". DailyMed. 30 January 2023. Archived from the original on 6 September 2023. Retrieved 5 July 2023.
- ^ "Metronidazole Vaginal Gel, 0.75%- metronidazole gel". DailyMed. 17 June 2023. Archived from the original on 6 September 2023. Retrieved 5 July 2023.
- ^ a b c d e "Flagyl, Flagyl ER (metronidazole) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 7 April 2014. Retrieved 3 April 2014.
- ^ a b c d e Brayfield A, ed. (14 January 2014). "Metronidazole". Martindale: The Complete Drug Reference. Pharmaceutical Press. Retrieved 3 April 2014.[dead link]
- ^ Brayfield A, ed. (2017). Martindale: The Complete Drug Reference (39th ed.). London: Pharmaceutical Press. ISBN 978-0-85711-309-2.
- ^ a b c d e f g "Metronidazole". The American Society of Health-System Pharmacists. Archived from the original on 6 September 2015. Retrieved 31 July 2015.
- ^ McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. (March 2018). "Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA)". Clinical Infectious Diseases. 66 (7): e1–e48. doi:10.1093/cid/cix1085. PMC 6018983. PMID 29462280.
- ^ "Safety in Lactation: Metronidazole and tinidazole". SPS - Specialist Pharmacy Service. Archived from the original on 21 February 2020. Retrieved 22 February 2020.
- ^ Corey EJ (2013). Drug discovery practices, processes, and perspectives. Hoboken, N.J.: John Wiley & Sons. p. 27. ISBN 978-1-118-35446-9. Archived from the original on 8 September 2017.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Schmid G (28 July 2003). "Trichomoniasis treatment in women". Archived from the original on 1 August 2015. Retrieved 1 August 2015.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Metronidazole - Drug Usage Statistics". ClinCalc. Archived from the original on 13 April 2020. Retrieved 14 January 2024.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search