
Back ميريستيسين Arabic ميريستيسين AZB Miristicina Catalan Myristicin Czech Myristicin Danish Myristicin German Miristikino Esperanto Miristicina Spanish میریستیسین Persian Myristisiini Finnish
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 3-methoxy-4,5-methylenedioxy-allylbenzene; 5-methoxy-3,4-methylenedioxy-allylbenzene |
| Dependence liability | Unknown |
| Addiction liability | Low |
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.009.225 |
| Chemical and physical data | |
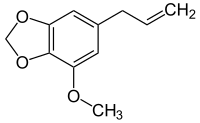
| Formula | C11H12O3 |
| Molar mass | 192.214 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Myristicin is a naturally occurring compound found in common herbs and spices, such as nutmeg.[1][2] It is an insecticide, and has been shown to enhance the effectiveness of other insecticides.[1][3]
When ingested, myristicin may produce hallucinogenic effects,[1][4] and can be converted to MMDMA in controlled chemical synthesis.[5] It interacts with many enzymes and signaling pathways in the body,[6][7] and may have dose-dependent cytotoxicity in living cells.[6] Myristicin is listed in the Hazardous Substances Data Bank.[1]
- ^ a b c d "Myristicin". PubChem, US National Library of Medicine. 13 May 2023. Retrieved 14 May 2023.
- ^ "Nutmeg". Drugs.com. 21 November 2022. Retrieved 14 May 2023.
- ^ Lichtenstein EP, Casida JE (1963). "Naturally Occurring Insecticides, Myristicin, an Insecticide and Synergist Occurring Naturally in the Edible Parts of Parsnips". Journal of Agricultural and Food Chemistry. 11 (5): 410–415. doi:10.1021/jf60129a017.
- ^ Stein U, Greyer H, Hentschel H (April 2001). "Nutmeg (myristicin) poisoning--report on a fatal case and a series of cases recorded by a poison information centre". Forensic Science International. 118 (1): 87–90. doi:10.1016/s0379-0738(00)00369-8. PMID 11343860.
- ^ Clark CR, DeRuiter J, Noggle FT (1996-01-01). "Analysis of 1-(3-Methoxy-4,5-Methylenedioxyphenyl)-2-Propanamine(MMDA)Derivatives Synthesized from Nutmeg Oil and 3-Methoxy-4,5-Methylenedioxybenzaldehyde". Journal of Chromatographic Science. 34 (1): 34–42. doi:10.1093/chromsci/34.1.34.
- ^ a b Lee BK, Kim JH, Jung JW, Choi JW, Han ES, Lee SH, et al. (May 2005). "Myristicin-induced neurotoxicity in human neuroblastoma SK-N-SH cells". Toxicology Letters. 157 (1): 49–56. doi:10.1016/j.toxlet.2005.01.012. PMID 15795093.
- ^ Yang AH, He X, Chen JX, He LN, Jin CH, Wang LL, et al. (July 2015). "Identification and characterization of reactive metabolites in myristicin-mediated mechanism-based inhibition of CYP1A2". Chemico-Biological Interactions. 237: 133–40. doi:10.1016/j.cbi.2015.06.018. PMID 26091900.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search