
Back نالوكسيجيل Arabic Naloxégol French נאלוקסגול HE Naloxegol Hungarian ナロキセゴール Japanese ନାଲୋକ୍ସେଗୋଲ OR Naloxegol Romanian Налоксегол Russian Naloxegol Vietnamese
 | |
| Clinical data | |
|---|---|
| Trade names | Movantik, Moventig |
| Other names | NKTR-118 |
| AHFS/Drugs.com | movantik |
| License data | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | ~4.2% |
| Metabolism | Liver (CYP3A) |
| Elimination half-life | 6–11 h |
| Excretion | Feces (68%), urine (16%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
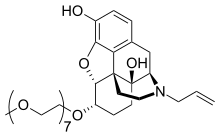
| Formula | C34H53NO11 |
| Molar mass | 651.794 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Naloxegol (INN; PEGylated naloxol;[3] trade names Movantik and Moventig) is a peripherally acting μ-opioid receptor antagonist developed by AstraZeneca, licensed from Nektar Therapeutics, for the treatment of opioid-induced constipation.[4] It was approved in 2014 in adult patients with chronic, non-cancer pain.[5] Doses of 25 mg were found safe and well tolerated for 52 weeks.[6] When given concomitantly with opioid analgesics, naloxegol reduced constipation-related side effects, while maintaining comparable levels of analgesia.[7]
The most common side effects are abdominal pain, diarrhea, nausea, flatulence, vomiting and headache. Patients often describe the above side effects to be similar to an instant withdrawal state brought on quickly rather than the 24 hours it may take to occur naturally. As a pure opioid antagonist Naloxegol has no potential for abuse.
Naloxegol was previously a Schedule II drug in the United States because of its chemical similarity to opium alkaloids. It was officially decontrolled on 23 January 2015. It was reclassified as a prescription drug after the FDA and DEA concluded that the impermeability of the blood–brain barrier to this compound made it non-habit-forming, and so without the potential for abuse.[8]
- ^ "Prescription medicines: registration of new chemical entities in Australia, 2016". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 10 April 2023.
- ^ "Health Canada New Drug Authorizations: 2015 Highlights". Health Canada. 4 May 2016. Retrieved 7 April 2024.
- ^ Seifert R, Wieland T, Mannhold R, Kubinyi H, Folkers G (17 July 2006). G Protein-Coupled Receptors as Drug Targets: Analysis of Activation and Constitutive Activity. John Wiley & Sons. p. 227. ISBN 978-3-527-60695-5. Retrieved 14 May 2012.
- ^ "Nektar | R&D Pipeline | Products in Development | CNS/Pain | Oral Naloxegol (NKTR-118) and Oral NKTR-119". Archived from the original on 2012-02-13. Retrieved 2012-05-14.
- ^ "FDA approves MOVANTIK™ (naloxegol) Tablets C-II for the treatment of opioid-induced constipation in adult patients with chronic non-cancer pain". 16 September 2014. Archived from the original on 2015-05-10.
- ^ Webster L, Chey WD, Tack J, Lappalainen J, Diva U, Sostek M (October 2014). "Randomised clinical trial: the long-term safety and tolerability of naloxegol in patients with pain and opioid-induced constipation" (PDF). Alimentary Pharmacology & Therapeutics. 40 (7): 771–9. doi:10.1111/apt.12899. PMID 25112584. S2CID 34286557.
- ^ Garnock-Jones KP (March 2015). "Naloxegol: a review of its use in patients with opioid-induced constipation". Drugs. 75 (4): 419–25. doi:10.1007/s40265-015-0357-2. PMID 25666542. S2CID 207488539.
- ^ "Schedules of Controlled Substances: Removal of Naloxegol From Control". www.deadiversion.usdoj.gov. Archived from the original on 2016-03-09. Retrieved 2016-02-27.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search