
Back Neutron Afrikaans Neutron ALS Neutrón AN نيوترون Arabic نيوطرون ARY নিউট্ৰন Assamese Neutrón AST Neytron Azerbaijani نوترون AZB Neutruons BAT-SMG
 | |
| Classification | Baryon |
|---|---|
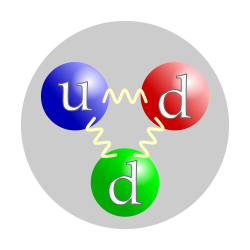
| Composition | 1 up quark, 2 down quarks |
| Statistics | Fermionic |
| Family | Hadron |
| Interactions | Gravity, weak, strong, electromagnetic |
| Symbol | n , n0 , N0 |
| Antiparticle | Antineutron |
| Theorized | Ernest Rutherford[1] (1920) |
| Discovered | James Chadwick[2] (1932) |
| Mass | 1.67492750056(85)×10−27 kg[3] 939.56542194(48) MeV[4] 1.00866491606(40) Da[5] |
| Mean lifetime | 878.4(5) s (free)[6] |
| Electric charge | 0 e (−2±8)×10−22 e (experimental limits)[7] |
| Electric dipole moment | < 1.8×10−26 e⋅cm (experimental upper limit) |
| Electric polarizability | 1.16(15)×10−3 fm3 |
| Magnetic moment | −0.96623650(23)×10−26 J·T−1[8] −1.04187563(25)×10−3 μB[8] −1.91304273(45) μN[8] |
| Magnetic polarizability | 3.7(20)×10−4 fm3 |
| Spin | 1/2 ħ |
| Isospin | −1/2 |
| Parity | +1 |
| Condensed | I(JP) = 1/2(1/2+) |
The neutron is a subatomic particle, symbol
n
or
n0
, that has no electric charge, and a mass slightly greater than that of a proton. The neutron was discovered by James Chadwick in 1932, leading to the discovery of nuclear fission in 1938, the first self-sustaining nuclear reactor (Chicago Pile-1, 1942) and the first nuclear weapon (Trinity, 1945).
Neutrons are found, together with a similar number of protons in the nuclei of atoms. Atoms of a chemical element that differ only in neutron number are called isotopes. Free neutrons are produced copiously in nuclear fission and fusion. They are a primary contributor to the nucleosynthesis of chemical elements within stars through fission, fusion, and neutron capture processes. Neutron stars, formed from massive collapsing stars, consist of neutrons at the density of atomic nuclei but a total mass more than the Sun.
Neutron properties and interactions are described by nuclear physics. Neutrons are not elementary particles; each is composed of three quarks. A free neutron spontaneously decays to a proton, an electron, and an antineutrino, with a mean lifetime of about 15 minutes.
The neutron is essential to the production of nuclear power. Dedicated neutron sources like neutron generators, research reactors and spallation sources produce free neutrons for use in irradiation and in neutron scattering experiments. Free neutrons do not directly ionize atoms, but they do indirectly cause ionizing radiation, so they can be a biological hazard, depending on dose. A small natural "neutron background" flux of free neutrons exists on Earth, caused by cosmic rays, and by the natural radioactivity of spontaneously fissionable elements in the Earth's crust.
- ^ Ernest Rutherford Archived 2011-08-03 at the Wayback Machine. Chemed.chem.purdue.edu. Retrieved on 2012-08-16.
- ^ 1935 Nobel Prize in Physics Archived 2017-10-03 at the Wayback Machine. Nobelprize.org. Retrieved on 2012-08-16.
- ^ "2022 CODATA Value: neutron mass". The NIST Reference on Constants, Units, and Uncertainty. NIST. May 2024. Retrieved 2024-05-18.
- ^ "2022 CODATA Value: neutron mass energy equivalent in MeV". The NIST Reference on Constants, Units, and Uncertainty. NIST. May 2024. Retrieved 2024-05-18.
- ^ "2022 CODATA Value: neutron mass in u". The NIST Reference on Constants, Units, and Uncertainty. NIST. May 2024. Retrieved 2024-05-18.
- ^ Zyla, P. A. (2020). "n MEAN LIFE". PDG Live: 2020 Review of Particle Physics. Particle Data Group. Archived from the original on 17 January 2021. Retrieved 25 February 2021.
- ^ Cite error: The named reference
PDGLIVEwas invoked but never defined (see the help page). - ^ a b c Mohr, P.J.; Taylor, B.N. and Newell, D.B. (2014), "The 2014 CODATA Recommended Values of the Fundamental Physical Constants" Archived 2013-10-09 at the Wayback Machine (Web Version 7.0). The database was developed by J. Baker, M. Douma, and S. Kotochigova. (2014). National Institute of Standards and Technology, Gaithersburg, Maryland 20899.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search