
Back Nikotienamied-adenien-dinukleotiedfosfaat Afrikaans فوسفات ثنائي نيوكليوتيد الأدينين وأميد النيكوتين Arabic نیکوتینآمید آدنین دینوکلئوتید فوسفات AZB НАДФ Bulgarian নিকোটিনামাইড অ্যাডেনিন ডাইনিউক্লিয়টাইড ফসফেট Bengali/Bangla Nikotinamid adenin dinukleotid-fosfat BS Fosfat de dinucleòtid de nicotinamida i adenina Catalan Nikotinamidadenindinukleotidfosfát Czech Nikotinamid-adenin-dinukleotidfosfat Danish Nicotinamidadenindinukleotidphosphat German
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.163 |
| MeSH | NADP |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H29N7O17P3 | |
| Molar mass | 744.416 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
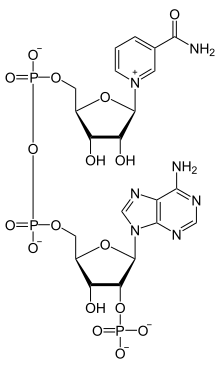
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP+ or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NADPH as a reducing agent ('hydrogen source'). NADPH is the reduced form, whereas NADP+ is the oxidized form. NADP+ is used by all forms of cellular life.[1]
NADP+ differs from NAD+ by the presence of an additional phosphate group on the 2' position of the ribose ring that carries the adenine moiety. This extra phosphate is added by NAD+ kinase and removed by NADP+ phosphatase.[2]
- ^ Spaans SK, Weusthuis RA, van der Oost J, Kengen SW (2015). "NADPH-generating systems in bacteria and archaea". Frontiers in Microbiology. 6: 742. doi:10.3389/fmicb.2015.00742. PMC 4518329. PMID 26284036.
- ^ Kawai S, Murata K (April 2008). "Structure and function of NAD kinase and NADP phosphatase: key enzymes that regulate the intracellular balance of NAD(H) and NADP(H)". Bioscience, Biotechnology, and Biochemistry. 72 (4): 919–30. doi:10.1271/bbb.70738. PMID 18391451.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search