
Back Nonilfenol Catalan Nonylfenol Czech Nonylphenole German Nonilfenol Spanish Nonilfenol Basque Nonylphénol French Nonilfenol Galician Nonil-fenol Hungarian Nonilfenolo Italian ノニルフェノール Japanese
| |
| Names | |
|---|---|
| IUPAC name
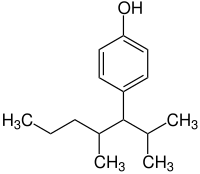
4-(2,4-dimethylheptan-3-yl)phenol
| |
| Other names
Phenol, nonyl-
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C15H24O | |
| Molar mass | 220.35 g/mol |
| Appearance | Light yellow viscous liquid with phenolic smell [1] |
| Density | 0.953 |
| Melting point | −8 to 2 °C (18 to 36 °F; 265 to 275 K) |
| Boiling point | 293 to 297 °C (559 to 567 °F; 566 to 570 K) |
| 6 mg/L (pH 7) | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
low level endrocrine disruptor |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nonylphenols are a family of closely related organic compounds composed of phenol bearing a 9 carbon-tail. Nonylphenols can come in numerous structures, all of which may be considered alkylphenols. They are used in manufacturing antioxidants, lubricating oil additives, laundry and dish detergents, emulsifiers, and solubilizers.[2] They are used extensively in epoxy formulation in North America[3][4] but its use has been phased out in Europe.[5] These compounds are also precursors to the commercially important non-ionic surfactants alkylphenol ethoxylates and nonylphenol ethoxylates, which are used in detergents, paints, pesticides, personal care products, and plastics. Nonylphenol has attracted attention due to its prevalence in the environment and its potential role as an endocrine disruptor and xenoestrogen, due to its ability to act with estrogen-like activity.[6] The estrogenicity and biodegradation heavily depends on the branching of the nonyl sidechain.[7][8][9] Nonylphenol has been found to act as an agonist of the GPER (GPR30).[10]
- ^ Record of Nonylphenol, mixed isomers in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 6 April 2011.
- ^ Cite error: The named reference
Soareswas invoked but never defined (see the help page). - ^ "Epoxy Nonyl Phenol Alert is it in your epoxy?". www.epoxyproducts.com. Retrieved 2021-03-18.
- ^ "para nonyl phenol" (PDF). squarespace.
- ^ "No, no, nonyl(phenol)". Healthy Building Network. Retrieved 2021-03-18.
- ^ Cite error: The named reference
mariawas invoked but never defined (see the help page). - ^ Guenther, Klaus; Kleist, Einhard; Thiele, Bjoern (2005-12-10). "Estrogen-active nonylphenols from an isomer-specific viewpoint: a systematic numbering system and future trends". Analytical and Bioanalytical Chemistry. 384 (2): 542–546. doi:10.1007/s00216-005-0181-8. ISSN 1618-2642. PMID 16341851. S2CID 39833642.
- ^ Gabriel FL, Routledge EJ, Heidelberger A, Rentsch D, Guenther K, Giger W, et al. (2008). "Isomer-specific degradation and endocrine disrupting activity of nonylphenols" (PDF). Environ Sci Technol. 42 (17): 6399–408. Bibcode:2008EnST...42.6399G. doi:10.1021/es800577a. PMID 18800507.
- ^ Lu, Zhijiang; Gan, Jay (2014-01-21). "Isomer-Specific Biodegradation of Nonylphenol in River Sediments and Structure-Biodegradability Relationship". Environmental Science & Technology. 48 (2): 1008–1014. Bibcode:2014EnST...48.1008L. doi:10.1021/es403950y. ISSN 0013-936X. PMID 24345275.
- ^ Prossnitz, Eric R.; Barton, Matthias (2014). "Estrogen biology: New insights into GPER function and clinical opportunities". Molecular and Cellular Endocrinology. 389 (1–2): 71–83. doi:10.1016/j.mce.2014.02.002. ISSN 0303-7207. PMC 4040308. PMID 24530924.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search