
Back أوسيلتاميفير Arabic اوسلتامیویر AZB ཨོ་སེལ་ཏཱ་མི་ཝིར། Tibetan Oseltamivir Czech Tamiflu Danish Oseltamivir German Οσελταμιβίρη Greek Oseltamiviro Esperanto Oseltamivir Spanish اسلتامیویر Persian
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ɒsəlˈtæmɪvɪər/ |
| Trade names | Tamiflu, others |
| Other names | GS-4104 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a699040 |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth |
| Drug class | Neuraminidase inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | >80%[4] |
| Protein binding | 42% (parent drug), 3% (active metabolite)[4] |
| Metabolism | Liver, to oseltamivir carboxylate[4] |
| Elimination half-life | 1–3 hours, 6–10 hours (active metabolite)[4] |
| Excretion | Urine (>90% as oseltamivir carboxylate), faeces[4] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
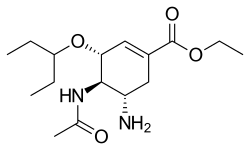
| Formula | C16H28N2O4 |
| Molar mass | 312.410 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Oseltamivir, sold under the brand name Tamiflu among others, is an antiviral medication used to treat and prevent influenza A and influenza B, viruses that cause the flu.[5] Many medical organizations recommend it in people who have complications or are at high risk of complications within 48 hours of first symptoms of infection.[6] They recommend it to prevent infection in those at high risk, but not the general population.[6] The Centers for Disease Control and Prevention (CDC) recommends that clinicians use their discretion to treat those at lower risk who present within 48 hours of first symptoms of infection.[6][7][8] It is taken by mouth, either as a pill or liquid.[5]
Recommendations regarding oseltamivir are controversial as are criticisms of the recommendations.[6][9][10][11] A 2014 Cochrane Review concluded that oseltamivir does not reduce hospitalizations, and that there is no evidence of reduction in complications of influenza.[11] Two meta-analyses have concluded that benefits in those who are otherwise healthy do not outweigh its risks.[12][13] They also found little evidence regarding whether treatment changes the risk of hospitalization or death in high risk populations.[12][13] However, another meta-analysis found that oseltamivir was effective for prevention of influenza at the individual and household levels.[14]
Common side effects include vomiting, diarrhea, headache, and trouble sleeping.[5] Other side effects may include psychiatric symptoms and seizures.[5][15][16] In the United States it is recommended for influenza infection during pregnancy.[1] It has been taken by a small number of pregnant women without signs of problems.[1] Dose adjustment may be needed in those with kidney problems.[5]
Oseltamivir was approved for medical use in the US in 1999.[5] It was the first neuraminidase inhibitor available by mouth.[17] It is on the World Health Organization's List of Essential Medicines but was downgraded to "complementary" status in 2017.[18][19][20] A generic version was approved in the US in 2016.[21][22] In 2022, it was the 205th most commonly prescribed medication in the United States, with more than 1 million prescriptions.[23][24]
- ^ a b c "Oseltamivir (Tamiflu) Use During Pregnancy". Drugs.com. Archived from the original on 9 September 2017. Retrieved 16 January 2017.
- ^ a b Roche Products Pty Limited. "Tamiflu (oseltamivir phosphate)". Australian Product Information. Archived from the original on 24 April 2023. Retrieved 9 January 2023.
- ^ "ALEMBIC OSELTAMIVIR, ALEMVIR , ALEMZY , OSELEMBIC, OSELTAMIVIR AGH, OSELTAMIVIR AGHL, OSELTAMIVIR APPL , TAMIRAC (Alembic Pharmaceuticals Australia Pty Ltd)". Therapeutic Goods Administration (TGA). 5 December 2022. Archived from the original on 18 March 2023. Retrieved 29 April 2023.
- ^ a b c d e Davies BE (April 2010). "Pharmacokinetics of oseltamivir: an oral antiviral for the treatment and prophylaxis of influenza in diverse populations". The Journal of Antimicrobial Chemotherapy. 65 (Suppl 2): ii5 – ii10. doi:10.1093/jac/dkq015. PMC 2835511. PMID 20215135.
- ^ a b c d e f "Oseltamivir Phosphate Monograph for Professionals". The American Society of Health-System Pharmacists. Archived from the original on 13 May 2016. Retrieved 8 January 2017.
- ^ a b c d "CDC Recommendations for Influenza Antiviral Medications Remain Unchanged". U.S. Centers for Disease Control and Prevention (CDC). 10 April 2014. Archived from the original on 18 January 2017. Retrieved 16 January 2017.
- ^ European Centre for Disease Prevention and Control (2 June 2014). "New and updated evaluations of neuraminidase inhibitors for preventing and treating influenza published". Archived from the original on 2 November 2014.
- ^ "Amantadine, oseltamivir and zanamivir for the treatment of influenza". National Institute for Health and Care Excellence (NICE). 25 February 2009. Archived from the original on 18 January 2017. Retrieved 16 January 2017.
- ^ "IDSA Continues to Recommend Antivirals for Influenza". Archived from the original on 24 April 2014. Retrieved 24 April 2014.
- ^ Brownlee S (19 February 2013). "Tamiflu: Myth and Misconception". The Atlantic. Archived from the original on 29 December 2014. Retrieved 7 December 2014.
- ^ a b Butler D (April 2014). "Tamiflu report comes under fire". Nature. 508 (7497): 439–40. Bibcode:2014Natur.508..439B. doi:10.1038/508439a. PMID 24759392.
- ^ a b Michiels B, Van Puyenbroeck K, Verhoeven V, Vermeire E, Coenen S (2013). Jefferson T (ed.). "The value of neuraminidase inhibitors for the prevention and treatment of seasonal influenza: a systematic review of systematic reviews". PLOS ONE. 8 (4): e60348. Bibcode:2013PLoSO...860348M. doi:10.1371/journal.pone.0060348. PMC 3614893. PMID 23565231.
- ^ a b Ebell MH, Call M, Shinholser J (April 2013). "Effectiveness of oseltamivir in adults: a meta-analysis of published and unpublished clinical trials". Family Practice. 30 (2): 125–33. doi:10.1093/fampra/cms059. PMID 22997224.
- ^ Okoli GN, Otete HE, Beck CR, Nguyen-Van-Tam JS (9 December 2014). "Use of neuraminidase inhibitors for rapid containment of influenza: a systematic review and meta-analysis of individual and household transmission studies". PLOS ONE. 9 (12): e113633. Bibcode:2014PLoSO...9k3633O. doi:10.1371/journal.pone.0113633. PMC 4260958. PMID 25490762.
- ^ Wang K, Shun-Shin M, Gill P, Perera R, Harnden A (April 2012). Harnden A (ed.). "Neuraminidase inhibitors for preventing and treating influenza in children (published trials only)". The Cochrane Database of Systematic Reviews. 2012 (4): CD002744. doi:10.1002/14651858.CD002744.pub4. PMC 6599832. PMID 22513907.
- ^ Jefferson T, Jones M, Doshi P, Spencer EA, Onakpoya I, Heneghan CJ (April 2014). "Oseltamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments". BMJ. 348: g2545. doi:10.1136/bmj.g2545. PMC 3981975. PMID 24811411.
- ^ Agrawal R, Rewatkar PV, Kokil GR, Verma A, Kalra A (July 2010). "Oseltamivir: a first line defense against swine flu". Medicinal Chemistry. 6 (4): 247–251. doi:10.2174/1573406411006040247. PMID 20843284.
- ^ Cite error: The named reference
WHO TRS 1006was invoked but never defined (see the help page). - ^ Ebell MH (July 2017). "WHO downgrades status of oseltamivir". BMJ. 358: j3266. doi:10.1136/bmj.j3266. PMID 28701339. S2CID 206916214. Archived from the original on 29 April 2023. Retrieved 31 August 2020.
- ^ Cite error: The named reference
WHO21stwas invoked but never defined (see the help page). - ^ "The FDA approves first generic version of widely used influenza drug, Tamiflu". U.S. Food and Drug Administration (FDA). 4 August 2016. Archived from the original on 8 August 2016. Retrieved 6 August 2016.
- ^ "Drugs@FDA: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 30 November 2017. Retrieved 9 January 2020.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Oseltamivir Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search