Back بيكروتوكسين Arabic پیکروتوکسین AZB Picrotoxina Catalan Pikrotoxin Czech Picrotoxin German Picrotoxina Spanish Pikrotoksiini Finnish Picrotoxine French ピクロトキシン Japanese Picrotoxine Dutch
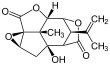
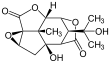
Picrotoxinin (left) and picrotin (right) | |||
| Clinical data | |||
|---|---|---|---|
| ATC code |
| ||
| Identifiers | |||
| CAS Number | |||
| PubChem CID | |||
| IUPHAR/BPS | |||
| DrugBank | |||
| ChemSpider | |||
| UNII | |||
| KEGG | |||
| ChEBI | |||
| ChEMBL | |||
| CompTox Dashboard (EPA) | |||
| ECHA InfoCard | 100.004.288 | ||
| Chemical and physical data | |||
| 3D model (JSmol) | |||
| |||
| |||
| | |||
Picrotoxin, also known as cocculin, is a poisonous crystalline plant compound. It was first isolated by the French pharmacist and chemist Pierre François Guillaume Boullay (1777–1869) in 1812.[1] The name "picrotoxin" is a combination of the Greek words "picros" (bitter) and "toxicon" (poison).[2] A mixture of two different compounds, picrotoxin occurs naturally in the fruit of the Anamirta cocculus plant, although it can also be synthesized chemically.
Due to its interactions with the inhibitory neurotransmitter GABA, picrotoxin acts as a stimulant and convulsant. It mainly impacts the central nervous system, causing seizures and respiratory paralysis in high enough doses.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search