
Back نازعة هيدروجين البيروفات Arabic Piruvat deshidrogenasa Catalan Pyruvatdehydrogenase Danish Pyruvatdehydrogenase E1 German Piruvato deshidrogenasa Spanish پیروات دهیدروژناز Persian Pyruvate déshydrogénase French Piruvato deshidroxenase Galician פירובט דהידרוגנאז HE Piruvat dehidrogenase ID
| pyruvate dehydrogenase (acetyl-transferring) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Crystallographic structure of pyruvate dehydrogenase (PDH). PH is a six domain dimer with α (blue), α’ (yellow), β (red), and β’ (teal) regions denoted by the different colors. Thiamine pyrophosphate (TPP) is shown in grey ball and stick form, two magnesium ions in purple undergoing metal ligation with the TPP, and two potassium ions in orange.[1] | |||||||||
| Identifiers | |||||||||
| EC no. | 1.2.4.1 | ||||||||
| CAS no. | 9014-20-4 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Pyruvate dehydrogenase is an enzyme that catalyzes the reaction of pyruvate and a lipoamide to give the acetylated dihydrolipoamide and carbon dioxide. The conversion requires the coenzyme thiamine pyrophosphate.
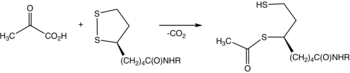
Pyruvate dehydrogenase is usually encountered as a component, referred to as E1, of the pyruvate dehydrogenase complex (PDC). PDC consists of other enzymes, referred to as E2 and E3. Collectively E1-E3 transform pyruvate, NAD+, coenzyme A into acetyl-CoA, CO2, and NADH. The conversion is crucial because acetyl-CoA may then be used in the citric acid cycle to carry out cellular respiration.[2] To distinguish between this enzyme and the PDC, it is systematically called pyruvate dehydrogenase (acetyl-transferring).
- ^ PDB: 1ni4; Ciszak EM, Korotchkina LG, Dominiak PM, Sidhu S, Patel MS (June 2003). "Structural basis for flip-flop action of thiamin pyrophosphate-dependent enzymes revealed by human pyruvate dehydrogenase". J. Biol. Chem. 278 (23): 21240–6. doi:10.1074/jbc.M300339200. hdl:2060/20030106063. PMID 12651851.
- ^ J. M. Berg; J. L. Tymoczko, L. Stryer (2007). Biochemistry (6th ed.). Freeman. ISBN 978-0-7167-8724-2.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search