Back ريزورسينول Arabic Rezorsin Azerbaijani رزورسینول AZB Resorcinol Catalan Resorcinol Czech Resorcin German Ρεσορκινόλη Greek Rezorcinolo Esperanto Resorcinol Spanish رزورسینول Persian
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Benzene-1,3-diol[1] | |||
| Other names
Resorcinol[1]
Resorcin m-Dihydroxybenzene 1,3-Benzenediol 1,3-Dihydroxybenzene 3-Hydroxyphenol m-Benzenediol | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.260 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| UN number | 2876 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H6O2 | |||
| Molar mass | 110.111 g/mol | ||
| Appearance | White solid[2] | ||
| Odor | Faint[2] | ||
| Density | 1.28 g/cm3, solid | ||
| Melting point | 110 °C (230 °F; 383 K) | ||
| Boiling point | 277 °C (531 °F; 550 K) | ||
| 110 g/100 mL at 20 °C | |||
| Vapor pressure | 0.0002 mmHg (25 °C)[2] | ||
| Acidity (pKa) | 9.15[3] | ||
| −67.26×10−6 cm3/mol | |||
Refractive index (nD)
|
1.578[4] | ||
| 2.07±0.02 D[5] | |||
| Thermochemistry | |||
Std enthalpy of
formation (ΔfH⦵298) |
-368.0 kJ·mol−1[4] | ||
Enthalpy of fusion (ΔfH⦵fus)
|
20.4 kJ·mol−1[4] | ||
| Pharmacology | |||
| D10AX02 (WHO) S01AX06 (WHO) | |||
| Hazards | |||
| GHS labelling: | |||
  
| |||
| H302, H313, H315, H318, H400 | |||
| P273, P280, P305+P351+P338 | |||
| Flash point | 127 °C; 261 °F; 400 K[2] | ||
| 608 °C (1,126 °F; 881 K)[4] | |||
| Explosive limits | 1.4%-?[2] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
none[2] | ||
REL (Recommended)
|
TWA 10 ppm (45 mg/m3) ST 20 ppm (90 mg/m3)[2] | ||
IDLH (Immediate danger)
|
N.D.[2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
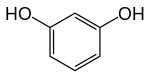
Resorcinol (or resorcin) is a phenolic compound. It is an organic compound with the formula C6H4(OH)2. It is one of three isomeric benzenediols, the 1,3-isomer (or meta-isomer). Resorcinol crystallizes from benzene as colorless needles that are readily soluble in water, alcohol, and ether, but insoluble in chloroform and carbon disulfide.[6]
- ^ a b "Front Matter". Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 691. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ a b c d e f g h NIOSH Pocket Guide to Chemical Hazards. "#0543". National Institute for Occupational Safety and Health (NIOSH).
- ^ Gawron, O.; Duggan, M.; Grelechi, C. (1952). "Manometric Determination of Dissociation Constants of Phenols". Analytical Chemistry. 24 (6): 969–970. doi:10.1021/ac60066a013.
- ^ a b c d CRC handbook of chemistry and physics: a ready-reference book of chemical and physical data. William M. Haynes, David R. Lide, Thomas J. Bruno (2016-2017, 97th ed.). Boca Raton, Florida. 2016. ISBN 978-1-4987-5428-6. OCLC 930681942.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link) - ^ Lander, John J.; Svirbely, John J. Lander, W. J. (1945). "The Dipole Moments of Catechol, Resorcinol and Hydroquinone". Journal of the American Chemical Society. 67 (2): 322–324. doi:10.1021/ja01218a051.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ K. W. Schmiedel; D. Decker (2012). "Resorcinol". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a23_111.pub2. ISBN 978-3527306732.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search