
Back روفلوميلاست Arabic Roflumilast German Roflumilast Spanish Roflumilast French Roflumilast Italian ロフルミラスト Japanese 로플루밀라스트 Korean ରୋଫ୍ଲୁମିଲାଷ୍ଟ OR Roflumilast Polish Roflumilaste Portuguese
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Daxas, Daliresp, Zoryve, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a611034 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, topical |
| Drug class | PDE4 inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 79%[4][3][8][9] |
| Protein binding | 99%[4][3][8][9] |
| Metabolism | Hepatic via CYP1A2 & CYP3A4[4][3][8][9] |
| Elimination half-life | 17 hours (30 hours [active metabolite])[4][3][8][9] |
| Excretion | Urine (70%)[4][3][8][9] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.210.960 |
| Chemical and physical data | |
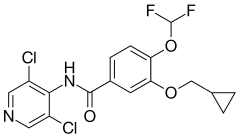
| Formula | C17H14Cl2F2N2O3 |
| Molar mass | 403.21 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Roflumilast, sold under the brand name Daxas among others, is a medication used for the treatment of chronic obstructive pulmonary disease,[4] plaque psoriasis,[5] seborrheic dermatitis,[6] and atopic dermatitis.[5] It acts as a selective, long-acting inhibitor of the enzyme phosphodiesterase-4 (PDE-4).[10] It has anti-inflammatory effects.[10][11][12]
It was approved for medical use in the European Union in 2010,[7] in the United States in 2011,[4] and in Canada in 2017.[1] It is available as a generic medication.[13]
- ^ a b "Daxas Product information". Health Canada. 20 July 2017. Retrieved 13 July 2024.
- ^ "Zoryve Product information". Health Canada. 7 November 2023. Retrieved 13 July 2024.
- ^ a b c d e f Cite error: The named reference
Daxas SmPCwas invoked but never defined (see the help page). - ^ a b c d e f g h "Daliresp- roflumilast tablet". DailyMed. 12 March 2020. Archived from the original on 25 March 2021. Retrieved 28 September 2020.
- ^ a b c Cite error: The named reference
Zoryve cream FDA labelwas invoked but never defined (see the help page). - ^ a b Cite error: The named reference
Zoryve foam FDA labelwas invoked but never defined (see the help page). - ^ a b "Daxas EPAR". European Medicines Agency. 17 September 2018. Archived from the original on 12 August 2020. Retrieved 28 September 2020.
- ^ a b c d e Cite error: The named reference
EMAwas invoked but never defined (see the help page). - ^ a b c d e Cite error: The named reference
MSRwas invoked but never defined (see the help page). - ^ a b Boswell-Smith V, Spina D (2007). "PDE4 inhibitors as potential therapeutic agents in the treatment of COPD-focus on roflumilast". International Journal of Chronic Obstructive Pulmonary Disease. 2 (2): 121–9. PMC 2695611. PMID 18044684.
- ^ Herbert C, Hettiaratchi A, Webb DC, Thomas PS, Foster PS, Kumar RK (May 2008). "Suppression of cytokine expression by roflumilast and dexamethasone in a model of chronic asthma". Clinical and Experimental Allergy. 38 (5): 847–56. doi:10.1111/j.1365-2222.2008.02950.x. PMID 18307529. S2CID 19050454.
- ^ Field SK (May 2008). "Roflumilast: an oral, once-daily selective PDE-4 inhibitor for the management of COPD and asthma". Expert Opinion on Investigational Drugs. 17 (5): 811–8. doi:10.1517/13543784.17.5.811. PMID 18447606. S2CID 73241684.
- ^ "2022 First Generic Drug Approvals". U.S. Food and Drug Administration (FDA). 3 March 2023. Archived from the original on 30 June 2023. Retrieved 30 June 2023.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search