
Back Natriumsesquicarbonat German Natria seskvikarbonato Esperanto Sesquicarbonato de sodio Spanish Sesquicarbonate de sodium French Տրինատրիումի հիդրոկարբոնատ Armenian セスキ炭酸ナトリウム Japanese Natriumsesquicarbonaat Dutch Soda śnieżna Polish Гидрокарбонат тринатрия Russian Natrijum seskvikarbonat Serbo-Croatian
This article needs additional citations for verification. (May 2017) |
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ECHA InfoCard | 100.007.802 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
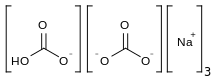
| Na3H(CO3)2·2H2O | |
| Appearance | white, needle-like |
| Density | 2.112 g/cm3 (dihydrate) |
| dihydrate 13 g/100 mL (0 °C) 42 g/100 mL (100 °C) | |
Refractive index (nD)
|
1.5073 (dihydrate) |
| Structure | |
| monoclinic (dihydrate) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium sesquicarbonate (systematic name: trisodium hydrogendicarbonate) Na3H(CO3)2 is a double salt of sodium bicarbonate and sodium carbonate (NaHCO3 · Na2CO3), and has a needle-like crystal structure. However, the term is also applied to an equimolar mixture of those two salts, with whatever water of hydration the sodium carbonate includes, supplied as a powder.
The dihydrate, Na3H(CO3)2 · 2H2O, occurs in nature as the evaporite mineral trona.[1]
Due to concerns about the toxicity of borax which was withdrawn as a cleaning and laundry product, sodium sesquicarbonate is sold in the European Union (EU) as "Borax substitute".[2] It is also known as one of the E number food additives E500(iii).
- ^ "Trona".
- ^ "Borax substitute – laundry booster, multi purpose cleaner, bath soak". Dri-Pak. 27 April 2015. Archived from the original on 2 July 2019. Retrieved 28 May 2017.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search