
Back ستركوبيلين Arabic استرکوبیلین AZB Sterkobilin BS Sterkobilin German Estercobilina Spanish استرکوبیلین Persian Stercobiline French Estercobilina Galician Sterkobilin Croatian Stercobilina Italian
| |
| Names | |
|---|---|
| IUPAC name
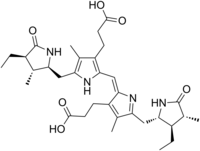
3-[(2E)-2-[ [3-(2-Carboxyethyl)-5- [(4-ethyl-3-methyl-5-oxo-pyrrolidin-2-yl) methyl]-4-methyl-1H-pyrrol-2-yl]methylidene]-5- [(3-ethyl-4-methyl-5-oxo-pyrrolidin-2-yl) methyl]-4-methyl-pyrrol-3-yl]propanoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.047.155 |
| MeSH | Stercobilin |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C33H46N4O6 | |
| Molar mass | 594.742 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Stercobilin is a tetrapyrrolic bile pigment and is one end-product of heme catabolism.[1][2] It is the chemical responsible for the brown color of human feces and was originally isolated from feces in 1932. Stercobilin (and related urobilin) can be used as a marker for biochemical identification of fecal pollution levels in rivers.[3]
- ^ Boron W, Boulpaep E. Medical Physiology: A cellular and molecular approach, 2005. 984-986. Elsevier Saunders, United States. ISBN 1-4160-2328-3
- ^ Kay IT, Weimer M, Watson CJ (1963). “The formation in vitro of stercobilin from bilirubin” Journal of Biological Chemistry. 238:1122-3. PMID 14031566
- ^ Lam, Ching-Wan; Lai, Chi-Kong; Chan, Yan-Wo (1 February 1998). "Simultaneous Fluorescence Detection of Fecal Urobilins and Porphyrins by Reversed-Phase High-Performance Thin-Layer Chromatography". Clinical Chemistry. 44 (2): 345–346. doi:10.1093/clinchem/44.2.345. PMID 9474036.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search