
Back ستريبينتول Arabic Stiripentol German استریپنتول Persian סטיריפנטול HE Stiripentolo Italian ଷ୍ଟିରିପେଣ୍ଟଲ OR Stiripentol Serbo-Croatian Stiripentol Serbian Stiripentol Turkish 司替戊醇 Chinese
 | |
| Clinical data | |
|---|---|
| Pronunciation | stir"i pen' tol |
| Trade names | Diacomit |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618069 |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.051.329 |
| Chemical and physical data | |
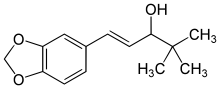
| Formula | C14H18O3 |
| Molar mass | 234.295 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Stiripentol, sold under the brand name Diacomit, is an anticonvulsant medication used for the treatment of Dravet syndrome - a serious genetic brain disorder.[5][6]
The most common side effects include loss of appetite, weight loss, insomnia (difficulty sleeping), drowsiness, ataxia (inability to co‑ordinate muscle movements), hypotonia (low muscle strength) and dystonia (muscle disorders).[5]
- ^ a b "Diacomit". Therapeutic Goods Administration (TGA). 13 December 2019. Retrieved 17 September 2021.
- ^ a b "AusPAR: Stiripentol". Therapeutic Goods Administration (TGA). 19 December 2019. Retrieved 17 September 2021.
- ^ "Diacomit 250mg hard capsules - Summary of Product Characteristics (SmPC)". (emc). 31 May 2019. Retrieved 8 November 2020.
- ^ Cite error: The named reference
Diacomit FDA labelwas invoked but never defined (see the help page). - ^ a b c Cite error: The named reference
Diacomit EPARwas invoked but never defined (see the help page). - ^ "Stiripentol Monograph for Professionals". Drugs.com. 31 August 2020. Retrieved 8 November 2020.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search