Back استیفنیک اسید AZB Styphninsäure German Ácido estífnico Spanish استیفنیک اسید Persian Acide styphnique French Acido stifnico Italian Stifnīnskābe Latvian/Lettish Ácido estífnico Portuguese Тринитрорезорцин Russian Stifninska kiselina Serbo-Croatian
| |||
| |||
| Names | |||
|---|---|---|---|
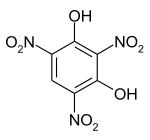
| Preferred IUPAC name
2,4,6-Trinitrobenzene-1,3-diol | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.001.306 | ||
PubChem CID
|
|||
| UNII | |||
| UN number | 0219 – Dry or wetted with < 20% water/alcohol 0394 – Wetted with >= 20% water/alcohol | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H3N3O8 | |||
| Molar mass | 245.11 g/mol | ||
| Density | 1.829 g/cm3 | ||
| Melting point | 180 °C (356 °F; 453 K) | ||
| Boiling point | decomposes | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Styphnic acid (from Greek stryphnos "astringent"[1]), or 2,4,6-trinitro-1,3-benzenediol, is a yellow astringent acid that forms hexagonal crystals. It is used in the manufacture of dyes, pigments, inks, medicines, and explosives such as lead styphnate. It is itself a low-sensitivity explosive, similar to picric acid, but explodes upon rapid heating.[2]
- ^ Alexander Senning (2006). Elsevier's Dictionary of Chemoetymology: The Whys and Whences of Chemical Nomenclature and Terminology, p. 375, at Google Books
- ^ Armarego, W.L.F.; Chai, C.L.L. (2003). Purification of Laboratory Chemicals. Butterworth-Heinemann. p. 353. ISBN 9780750675710. Retrieved 2015-05-20.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search