
Back كاشف تيبي Arabic آییراچ تبه AZB Tebbeovo činidlo Czech Tebbe-Reagenz German واکنشگر تبه Persian Tebben reagenssi Finnish Réactif de Tebbe French Reattivo di Tebbe Italian テッベ試薬 Japanese Tebbe-reagens Dutch
| |

| |
| Names | |
|---|---|
| IUPAC name
μ-Chloro[di(cyclopenta-2,4-dien-1-yl)]dimethyl(μ-methylene)titaniumaluminum
| |
| Other names
Tebbe reagent
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.157.162 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H18AlClTi | |
| Molar mass | 284.60 g/mol |
| Solubility in other solvents | toluene, benzene, dichloromethane, THF (low temperatures only) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tebbe's reagent is the organometallic compound with the formula (C5H5)2TiCH2ClAl(CH3)2. It is used in the methylidenation of carbonyl compounds, that is it converts organic compounds containing the R2C=O group into the related R2C=CH2 derivative.[1] It is a red solid that is pyrophoric in the air, and thus is typically handled with air-free techniques. It was originally synthesized by Fred Tebbe at DuPont Central Research.
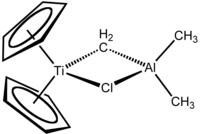
Tebbe's reagent contains two tetrahedral metal centers linked by a pair of bridging ligands. The titanium has two cyclopentadienyl ([C
5H
5]−
, or Cp) rings and aluminium has two methyl groups. The titanium and aluminium atoms are linked together by both a methylene bridge (-CH2-) and a chloride atom in a nearly square-planar (Ti–CH2–Al–Cl) geometry.[2] The Tebbe reagent was the first reported compound where a methylene bridge connects a transition metal (Ti) and a main group metal (Al).[3]
- ^ F. N. Tebbe, G. W. Parshall and G. S. Reddy (1978). "Olefin homologation with titanium methylene compounds". J. Am. Chem. Soc. 100 (11): 3611–3613. doi:10.1021/ja00479a061.
- ^ Thompson, Rick; Nakamaru-Ogiso, Eiko; Chen, Chun-Hsing; Pink, Maren; Mindiola, Daniel J. (2014). "Structural Elucidation of the Illustrious Tebbe Reagent". Organometallics. 33 (1): 429–432. doi:10.1021/om401108b.
- ^ Herrmann, W.A., "The Methylene Bridge" Advances in Organometallic Chemistry 1982, 20, 195–197.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search