
Back Tiosulfaat Afrikaans ثيوكبريتات Arabic Тиосулфат Bulgarian Tiosulfat Catalan Thiosíran Czech Органикăлла мар тиосульфат CV Thiosulfate German Θειοθειικό Greek Tiosulfato Spanish تیوسولفات Persian
| |

| |
| Names | |
|---|---|
IUPAC names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
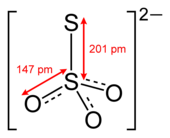
| S2O2−3 | |
| Molar mass | 112.12 g·mol−1 |
| Conjugate acid | Thiosulfuric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Thiosulfate (IUPAC-recommended spelling; sometimes thiosulphate in British English) is an oxyanion of sulfur with the chemical formula S2O2−3. Thiosulfate also refers to the compounds containing this anion, which are the salts of thiosulfuric acid, such as sodium thiosulfate Na2S2O3 and ammonium thiosulfate (NH4)2S2O3. Thiosulfate salts occur naturally. It rapidly dechlorinates water and is notable for its use to halt bleaching in the paper-making industry. Thiosulfate salts are mainly used in dying in textiles and the bleaching of natural substances.[2]
- ^ International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. pp. 139,329. Electronic version.
- ^ Cite error: The named reference
Ullmannwas invoked but never defined (see the help page).
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search