
Back ترانيلسيبرومين Arabic ترانیل سیپرومین AZB Tranylcypromin Welsh Tranylcypromin German Tranilcipromina Spanish ترانیل سیپرومین Persian Tranyylisypromiini Finnish Tranilcipromina Italian Tranylcypromin NB ଟ୍ରାନାଇଲସାଇପ୍ରୋମାଇନ OR
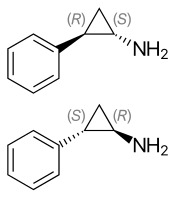 (1S,2R)-(−)-tranylcypromine (top), (1R,2S)-(+)-tranylcypromine (bottom) | |
| Clinical data | |
|---|---|
| Trade names | Parnate, many generics[1] |
| Other names | trans-2-phenylcyclopropylamine |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682088 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 50%[4] |
| Metabolism | Liver[5][6] |
| Elimination half-life | 2.5 hours[4] |
| Excretion | Urine, Feces[4] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.005.312 |
| Chemical and physical data | |
| Formula | C9H11N |
| Molar mass | 133.194 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
Tranylcypromine, sold under the brand name Parnate among others,[1] is a monoamine oxidase inhibitor (MAOI).[4][7] More specifically, tranylcypromine acts as nonselective and irreversible inhibitor of the enzyme monoamine oxidase (MAO).[4][7] It is used as an antidepressant and anxiolytic agent in the clinical treatment of mood and anxiety disorders, respectively. It is also effective in the treatment of ADHD.[8][9]
Tranylcypromine is a cyclopropylamine formed pro forma from the cyclization of amphetamine's side chain; therefore, it is classified as a substituted amphetamine.
- ^ a b "International brands for Tranylcypromine". Drugs.com. Retrieved 17 April 2016.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ a b c d e Williams DA (2007). "Antidepressants". In Foye WO, Lemke TL, Williams DA (eds.). Foye's Principles of Medicinal Chemistry. Hagerstwon, USA: Lippincott Williams & Wilkins. pp. 590–1. ISBN 978-0-7817-6879-5.
- ^ "Tranylcypromine". www.drugbank.ca. Retrieved 2019-12-06.
- ^ Baker GB, Urichuk LJ, McKenna KF, Kennedy SH (June 1999). "Metabolism of monoamine oxidase inhibitors". Cellular and Molecular Neurobiology. 19 (3): 411–26. doi:10.1023/a:1006901900106. PMID 10319194. S2CID 21380176.
- ^ a b Baldessarini RJ (2005). "17. Drug therapy of depression and anxiety disorders". In Brunton LL, Lazo JS, Parker KL (eds.). Goodman & Gilman's The Pharmacological Basis of Therapeutics. New York: McGraw-Hill. ISBN 978-0-07-142280-2.
- ^ Zametkin A, Rapoport JL, Murphy DL, Linnoila M, Ismond D. Treatment of hyperactive children with monoamine oxidase inhibitors. I. Clinical efficacy. Arch Gen Psychiatry. 1985 Oct;42(10):962-6. doi: 10.1001/archpsyc.1985.01790330042005. PMID: 3899047.
- ^ Levin GM. Attention-deficit hyperactivity disorder: the pharmacist's role. Am Pharm. 1995 Nov;NS35(11):10-20. PMID: 8533716.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search