
Back Trietanolamin Azerbaijani تریاتانولآمین AZB Triethanolamin German Trietanolamino Esperanto Trietanolamina Spanish Trietanolamina Basque تریاتانولآمین Persian Trietanoliamiini Finnish Triéthanolamine French Trietanolamin ID
| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
2,2′,2′′-Nitrilotri(ethan-1-ol)[1] | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| 1699263 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.773 |
| EC Number |
|
| KEGG | |
| MeSH | Biafine |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
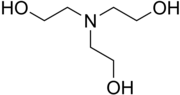
| N(CH2CH2OH)3 | |
| Molar mass | 149.190 g·mol−1 |
| Appearance | Colourless, viscous liquid |
| Odor | Ammoniacal |
| Density | 1.124 g/mL |
| Melting point | 21.60 °C; 70.88 °F; 294.75 K |
| Boiling point | 335.40 °C; 635.72 °F; 608.55 K |
| miscible | |
| log P | −0.988 |
| Vapor pressure | 1 Pa (at 20 °C) |
| Acidity (pKa) | 7.74[2] |
| UV-vis (λmax) | 280 nm |
Refractive index (nD)
|
1.485 |
| Thermochemistry | |
Heat capacity (C)
|
389 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−665.7 – −662.7 kJ mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
−3.8421 – −3.8391 MJ mol−1 |
| Pharmacology | |
| D03AX12 (WHO) | |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H319 | |
| P305+P351+P338 | |
| NFPA 704 (fire diamond) | |
| Flash point | 179 °C (354 °F; 452 K) |
| 325 °C (617 °F; 598 K) | |
| Explosive limits | 1.3–8.5% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
|
| Safety data sheet (SDS) | hazard.com |
| Related compounds | |
Related alkanols
|
|
Related compounds
|
Diethylhydroxylamine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Triethanolamine, or TEOA, is an organic compound with the chemical formula N(CH2CH2OH)3. It is a colourless, viscous liquid. It is both a tertiary amine and a triol. A triol is a molecule with three alcohol groups. Approximately 150,000 tonnes were produced in 1999.[3] It is a colourless compound although samples may appear yellow because of impurities.
- ^ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. P001–P004. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ Simond, M. R. (2012). "Dissociation Constants of Protonated Amines in Water at Temperatures from 293.15 K to 343.15 K". Journal of Solution Chemistry. 41: 130. doi:10.1007/s10953-011-9790-3. S2CID 95755026.
- ^ Frauenkron, Matthias; Melder, Johann-Peter; Ruider, Günther; Rossbacher, Roland; Höke, Hartmut. "Ethanolamines and Propanolamines". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_001. ISBN 978-3527306732.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search
