 structure | |
| Combination of | |
|---|---|
| secobarbital | short-acting barbiturate |
| amobarbital | intermediate-acting barbiturate |
| Clinical data | |
| Routes of administration | oral |
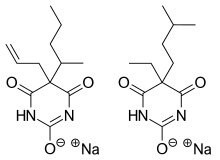
Tuinal was the brand name of a discontinued combination drug composed of two barbiturate salts (secobarbital sodium and amobarbital sodium) in equal proportions.
Tuinal was introduced as a sedative-hypnotic (sleeping pill) medication in the late 1940s by Eli Lilly. It was also used in obstetrics for childbirth.[1][2] It was produced in brightly colored half-reddish orange and half-turquoise blue gelatin capsule form (bullet-shaped Pulvules) for oral administration. Individual capsules contained 50 mg, 100 mg, or 200 mg of barbiturate salts. The combination of a short-acting barbiturate, Secobarbital, with an intermediate-acting barbiturate, Amobarbital, aimed to provide "a rapid yet prolonged hypnotic action".[3]
Eli Lilly has discontinued the manufacture of Tuinal in the United States due to the diminishing use of barbiturates (replaced by the benzodiazepine family of drugs) in outpatient treatment, and its widespread abuse.[4] Currently, Valleant Labs markets Secobarbital capsules only. Flynn Pharma of Ireland no longer manufactures Tuinal, Seconal or Amytal (amobarbital). Amytal has been discontinued, though sodium amytal injection form remains.
- ^ Waters AB (1947). "Pethidine In Labour". The British Medical Journal. 2 (4514): 71–72. doi:10.1136/bmj.2.4514.71-b. ISSN 0007-1447. JSTOR 20370143. PMC 2055200. PMID 20344014.
- ^ "Front Matter". The British Medical Journal. 1 (4539). 1948. ISSN 0007-1447. JSTOR 25361874.
- ^ "Front Matter". The American Journal of Nursing. 47 (5): 1–24. 1947. ISSN 0002-936X. JSTOR 3457169.
- ^ Mitchell M, Willingham EJ, Atkins WA (2012). Key K (ed.). The Gale Encyclopedia of Mental Health. Vol. 1 (3rd ed.). Detroit, MI: Gale eBooks. p. 171. ISBN 9781414490144. Retrieved November 4, 2022.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search
