
Back أوميفينوفير Arabic Umifenovir Czech Umifenovir German Umifenovir Spanish اومیفنوویر Persian Umifénovir French Umifenovir Italian ウミフェノビル Japanese Umifenovir Portuguese Umifenovir Romanian
You can help expand this article with text translated from the corresponding article in Russian. (June 2020) Click [show] for important translation instructions.
|
This article needs more reliable medical references for verification or relies too heavily on primary sources. (July 2020) |  |
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Arbidol |
| Other names | AR-1I9514 |
| Pregnancy category |
|
| Routes of administration | By mouth (hard capsules, tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 40% |
| Metabolism | Hepatic, CYP3A4[4] |
| Elimination half-life | 17–21 hours |
| Excretion | 40% excrete as unchanged umifenovir in feces (38.9%) and urine (0.12%)[3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.247.800 |
| Chemical and physical data | |
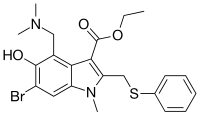
| Formula | C22H25BrN2O3S |
| Molar mass | 477.42 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Umifenovir, sold under the brand name Arbidol, is an antiviral medication for the treatment of influenza and COVID infections used in Russia[5] and China. The drug is manufactured by Pharmstandard (Russian: Фармстандарт). It is not approved by the U.S. Food and Drug Administration (FDA) for the treatment or prevention of influenza.[6]
Chemically, umifenovir features an indole core, functionalized at all but one positions with different substituents. The molecular groups of umifenovir - hydroxy, amino and carboxy - can interact to form various hydrogen-bonded synthons. Antiviral activity and acceptable cytotoxicity profiles [7][8] make it a promising candidate for further research as a potential therapeutic agent for the selective treatment of flavivirus infections.[9] Umifenovir is characterized by only one polymorphic form but can exist in the form of a large number of crystal solvates, the production of which depends on the medium and conditions of synthesis. In this case, the implementation of the crystal solvate or other solid form may be determined by the spatial structure and conformational equilibria in the saturated solution.[10][11] The drug has been shown in studies to inhibit viral entry into target cells [12] and stimulate the immune response.
- ^ a b "阿比朵尔抑制新冠,先声药业生产的吗?". 全民健康网 (in Chinese). Archived from the original on 17 June 2020. Retrieved 17 June 2020.
- ^ "ИНСТРУКЦИЯ ПО ПРИМЕНЕНИЮ АРПЕТОЛ (ARPETOL)". Vidal (in Belarusian). Archived from the original on 26 January 2018. Retrieved 17 June 2020.
- ^ "Full Prescribing Information: Arbidol (umifenovir) film-coated tablets 50 and 100 mg: Corrections and Additions". State Register of Medicines (in Russian). Open joint-stock company "Pharmstandard-Tomskchempharm". Retrieved 3 June 2015.[permanent dead link]
- ^ Deng P, Zhong D, Yu K, Zhang Y, Wang T, Chen X (April 2013). "Pharmacokinetics, metabolism, and excretion of the antiviral drug arbidol in humans". Antimicrobial Agents and Chemotherapy. 57 (4): 1743–1755. doi:10.1128/AAC.02282-12. PMC 3623363. PMID 23357765. S2CID 9534025.
- ^ Leneva IA, Russell RJ, Boriskin YS, Hay AJ (February 2009). "Characteristics of arbidol-resistant mutants of influenza virus: implications for the mechanism of anti-influenza action of arbidol". Antiviral Research. 81 (2): 132–140. doi:10.1016/j.antiviral.2008.10.009. PMID 19028526.
- ^ "FDA Approved Drugs for Influenza". U.S. Food and Drug Administration. 8 December 2022.
- ^ Leneva I, Kartashova N, Poromov A, Gracheva A, Korchevaya E, Glubokova E, et al. (August 2021). "Antiviral Activity of Umifenovir In Vitro against a Broad Spectrum of Coronaviruses, Including the Novel SARS-CoV-2 Virus". Viruses. 13 (8): 1665. doi:10.3390/v13081665. PMC 8402645. PMID 34452529.
- ^ Haviernik J, Štefánik M, Fojtíková M, Kali S, Tordo N, Rudolf I, et al. (April 2018). "Arbidol (Umifenovir): A Broad-Spectrum Antiviral Drug That Inhibits Medically Important Arthropod-Borne Flaviviruses". Viruses. 10 (4): 184. doi:10.3390/v10040184. PMC 5923478. PMID 29642580.
- ^ Qian X, Qi Z (June 2022). "Mosquito-Borne Flaviviruses and Current Therapeutic Advances". Viruses. 14 (6): 1226. doi:10.3390/v14061226. PMC 9229039. PMID 35746697.
- ^ Eventova VA, Belov KV, Efimov SV, Khodov IA (January 2023). "Conformational Screening of Arbidol Solvates: Investigation via 2D NOESY". Pharmaceutics. 15 (1): 226. doi:10.3390/pharmaceutics15010226. PMC 9865235. PMID 36678855.
- ^ Belov KV, Dyshin AA, Khodov IA (March 2024). "Conformational analysis of arbidol in supercritical carbon Dioxide: Insights into 'opened' and 'closed' conformer groups". Journal of Molecular Liquids. 397: 124074. doi:10.1016/j.molliq.2024.124074. S2CID 267232952.
- ^ Kadam RU, Wilson IA (January 2017). "Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol". Proceedings of the National Academy of Sciences of the United States of America. 114 (2): 206–214. Bibcode:2017PNAS..114..206K. doi:10.1073/pnas.1617020114. PMC 5240704. PMID 28003465.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search