Back فانيلين Arabic وانیلین AZB Ванілін Byelorussian Ванилин Bulgarian Vanil·lina Catalan Vanilin Czech Vanillin Danish Vanillin German Βανιλίνη Greek Vanilino Esperanto
| |||

| |||
| Names | |||
|---|---|---|---|
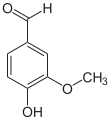
| Preferred IUPAC name
4-Hydroxy-3-methoxybenzaldehyde | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| 472792 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.060 | ||
| EC Number |
| ||
| 3596 | |||
| KEGG | |||
| MeSH | vanillin | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C8H8O3 | |||
| Molar mass | 152.149 g·mol−1 | ||
| Appearance | White solid | ||
| Odor | Vanilla, sweet, balsamic, pleasant | ||
| Density | 1.056 g/cm3[3] | ||
| Melting point | 81 °C (178 °F; 354 K)[3] | ||
| Boiling point | 285 °C (545 °F; 558 K)[3] | ||
| 10 g/L | |||
| log P | 1.208 | ||
| Vapor pressure | >1 Pa | ||
| Acidity (pKa) | 7.781 | ||
| Basicity (pKb) | 6.216 | ||
| Structure | |||
| Monoclinic | |||
| Thermochemistry | |||
Std enthalpy of
combustion (ΔcH⦵298) |
−3.828 MJ/mol | ||
| Hazards | |||
| GHS labelling: | |||

| |||
| Warning | |||
| H302, H317, H319 | |||
| P280, P305+P351+P338 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 147 °C (297 °F; 420 K) | ||
| Safety data sheet (SDS) | ICSC 1740 | ||
| Related compounds | |||
Related compounds
|
Anisaldehyde Apocynin Eugenol Phenol Vanillyl alcohol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Vanillin is an organic compound with the molecular formula C8H8O3. It is a phenolic aldehyde. Its functional groups include aldehyde, hydroxyl, and ether. It is the primary component of the extract of the vanilla bean. Synthetic vanillin is now used more often than natural vanilla extract as a flavoring in foods, beverages, and pharmaceuticals.
Vanillin and ethylvanillin are used by the food industry; ethylvanillin is more expensive, but has a stronger note. It differs from vanillin by having an ethoxy group (−O−CH2CH3) instead of a methoxy group (−O−CH3).
Natural vanilla extract is a mixture of several hundred different compounds in addition to vanillin. Artificial vanilla flavoring is often a solution of pure vanillin, usually of synthetic origin. Because of the scarcity and expense of natural vanilla extract, synthetic preparation of its predominant component has long been of interest. The first commercial synthesis of vanillin began with the more readily available natural compound eugenol (4-allyl-2-methoxyphenol). Today, artificial vanillin is made either from guaiacol or lignin.
Lignin-based artificial vanilla flavoring is alleged to have a richer flavor profile than that from guiacol-based artificial vanilla; the difference is due to the presence of acetovanillone, a minor component in the lignin-derived product that is not found in vanillin synthesized from guaiacol.[4]
- ^ a b Field, Simon Quellen. "Vanillin". sci-toys.com.
- ^ CID 1183 from PubChem.
- ^ a b c Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. p. 3.310. ISBN 978-1-4987-5429-3.
- ^ According to Esposito 1997, blind taste-testing panels cannot distinguish between the flavors of synthetic vanillin from lignin and those from guaicol, but can distinguish the odors of these two types of synthetic vanilla extracts. Guaiacol vanillin, adulterated with acetovanillone, has an odor indistinguishable from lignin vanillin.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search